Biopesticides are one aspect of biological control. The active ingredients in biopesticides include microorganisms (microbes), plant extracts, and naturally-occurring chemicals (like potassium bicarbonate). As a result, some of the ways they control pests (their modes of action or MOAs) are different from conventional, synthetic chemical pesticides. Also, many of them have several MOAs, and not all MOAs apply to all pests listed on the label. If a biopesticide contains live microbes, and especially if its MOA requires the microbes to stay alive on the plant for some period of time after application, this also has important implications for how the product is stored and applied. Understanding the mode of action of a product will help you get the most out of it.
I like to break down biopesticide MOAs into the following categories:
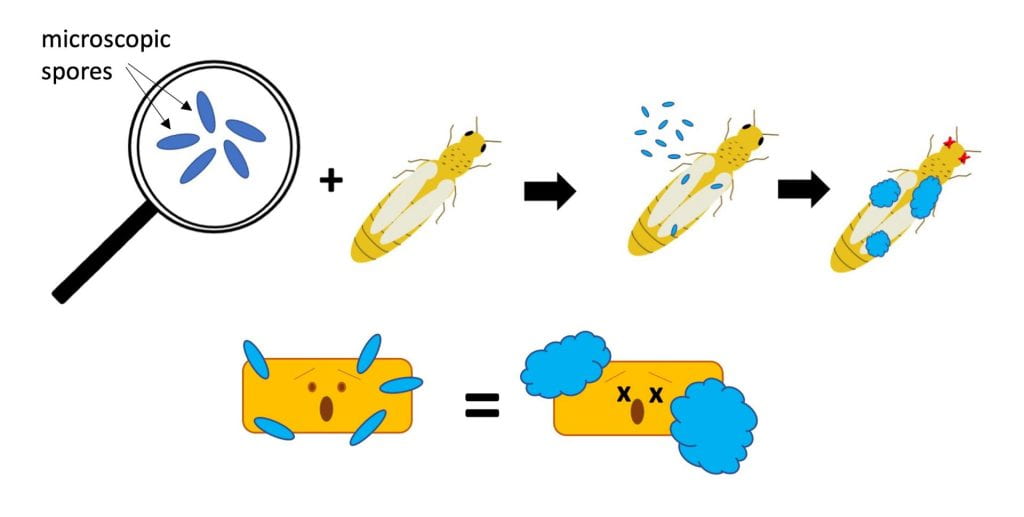
Eat – live microbe grows on/in pest
Biopesticides with this MOA can work against insect pests (e.g., products that contain Beauveria bassiana) or plant diseases (e.g. Contans, which contains Paraconionthyrium minitans strain CON/M/91-08). Many biopesticides with this MOA contain fungal spores. These spores will germinate once they land on the insect or disease-causing pathogen, and may have temperature and/or humidity requirements for germination. Make sure you store the product correctly, confirm compatibility with other products before tank mixing or applying, and apply under recommended environmental conditions.
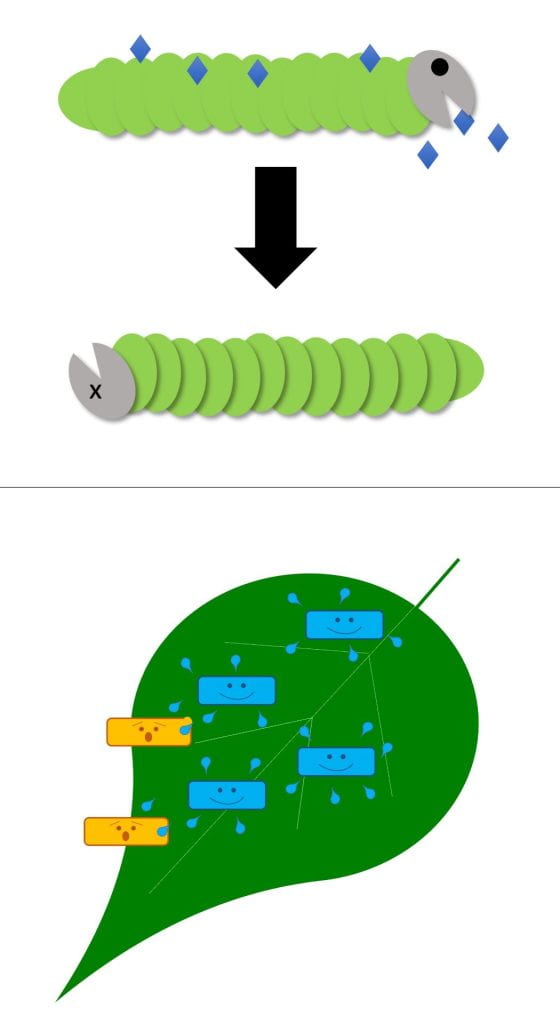
Poison – biopesticide (or its products) kills the pest directly
Biopesticides with this MOA can work against insect pests (like products containing Bacillus thuringiensis) or plant diseases (e.g., Double Nickel containing Bacillus amyloliquefacies strain D747, or products containing potassium bicarbonate). Obviously, potassium bicarbonate products do not contain live microbes. Some biopesticides that poison pests do have live microbes that continue to produce antimicrobial products after they are applied. Others work because of the compounds the microbes produced while the biopesticide was being made.
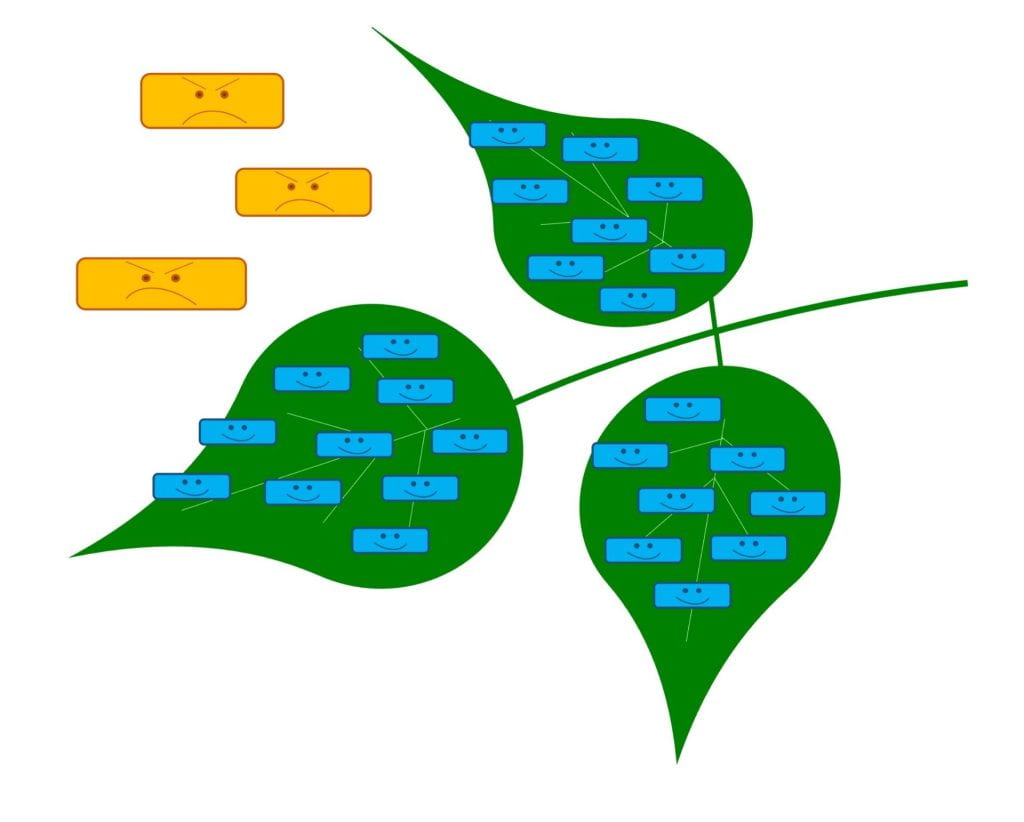
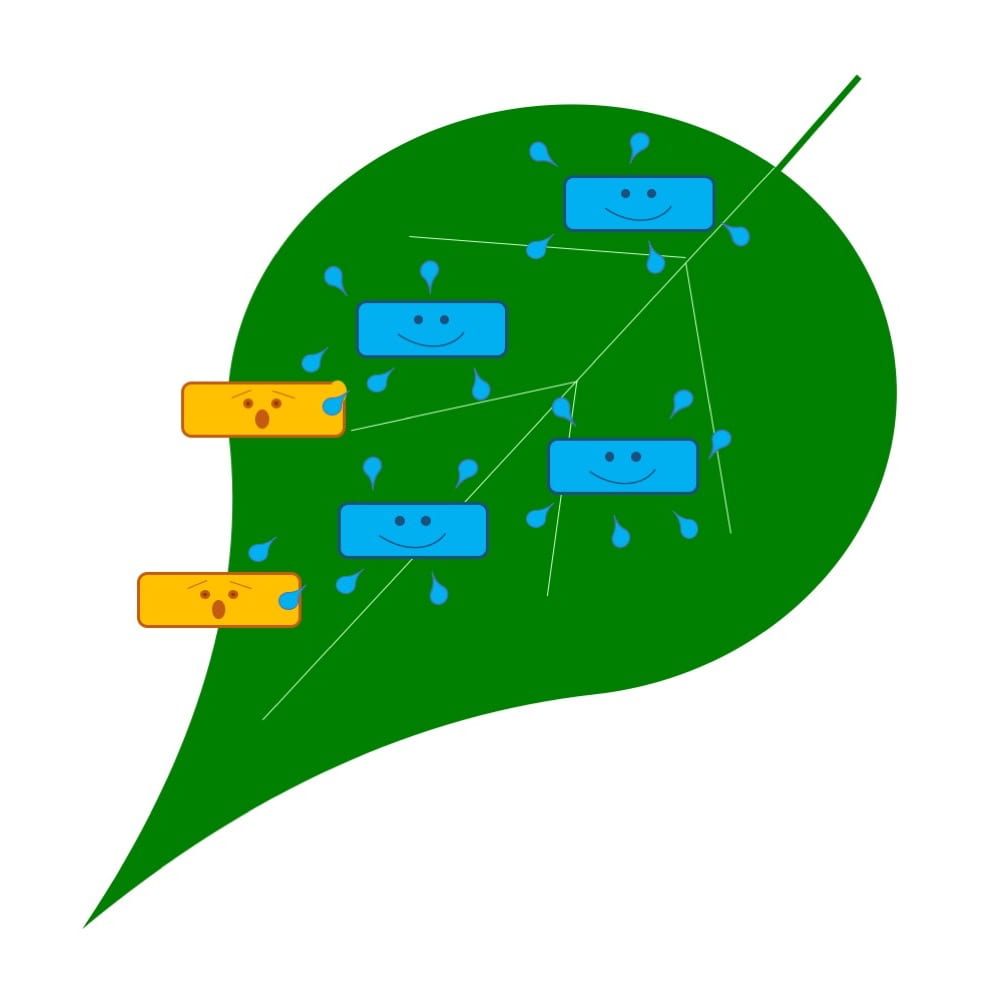
Keep out – live microbe grows on plant, leaving no room for pests
Biopesticides with this MOA can work against plant disease (e.g., Actinovate which contains Streptomyces lydicus WYEC 108, or Serifel, which contains Bacillus amyloliquefaciens strain MBI 600) and may be bacteria or fungi. The microbes in biopesticides with this MOA must be alive when applied and need to be able to grow on the part of the plant that is being protected.
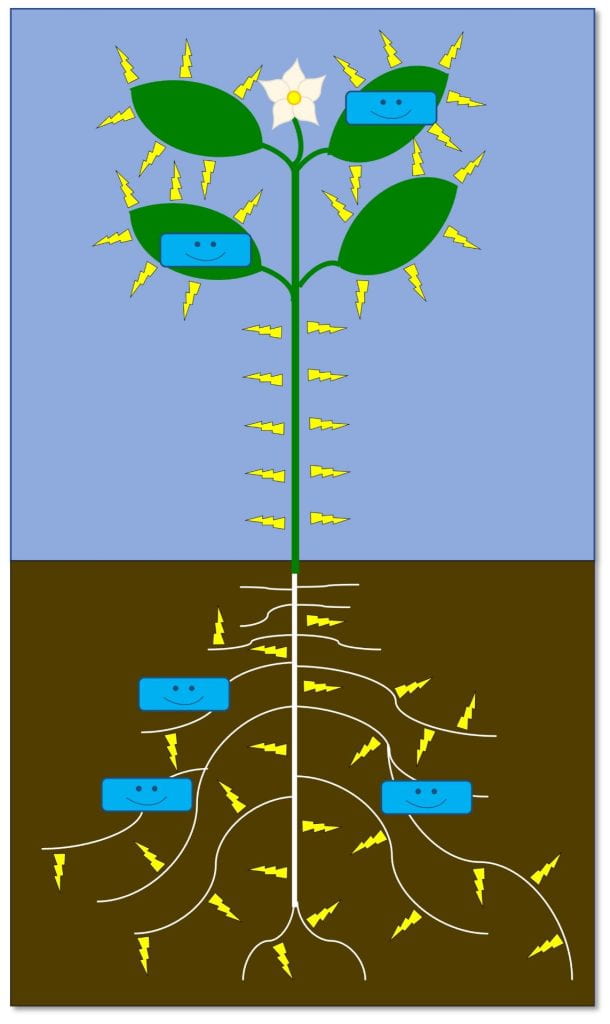
Turn on resistance – turns on the plant’s defenses before pest attacks
As far as I know, these biopesticides only work against plant diseases, but as new products are developed, or as we learn more about existing biopesticides, this may change. Some examples include Regalia (giant knotweed extract) and Lifeguard WG (Bacillus mycoides isolate J). Some of these products contain live microbes that need to stay alive (like LifeGard), while others do not. These biopesticides need to be applied before infection.
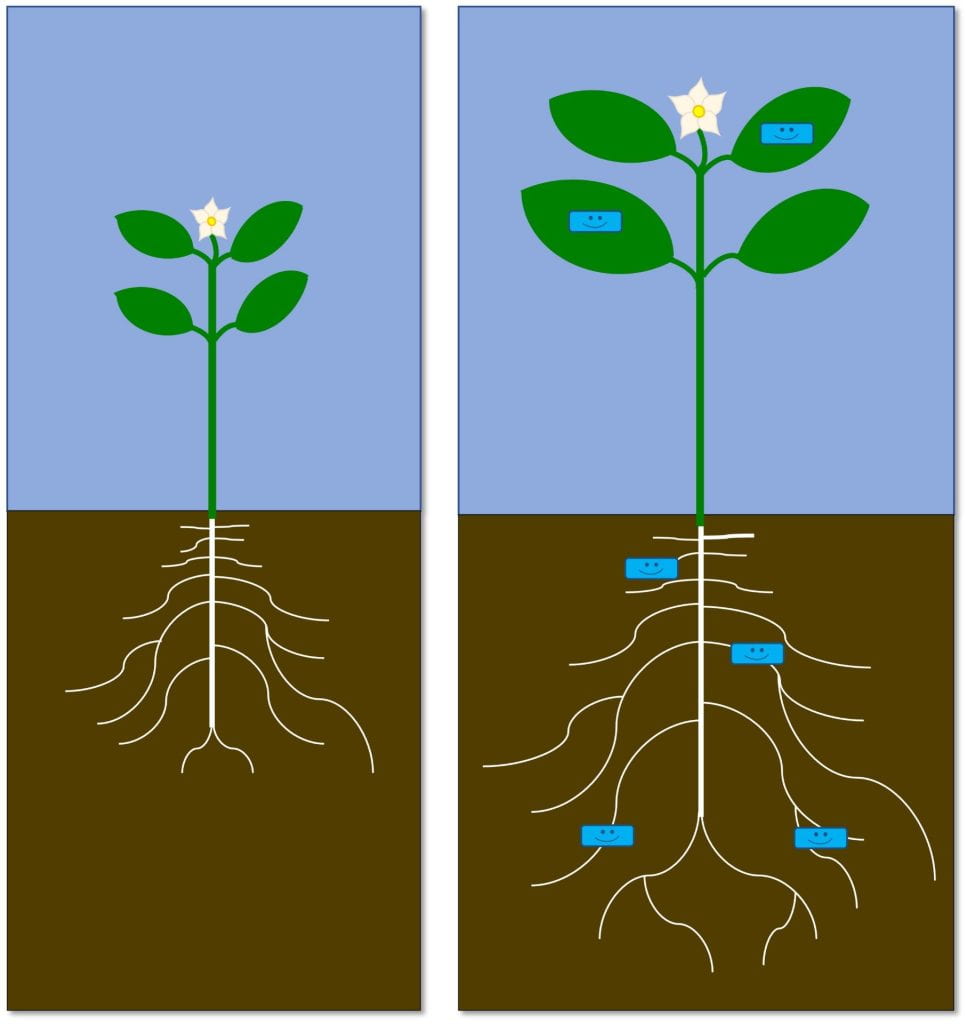
Grow strong plants – makes plant stronger, healthier, more resilient
These biopesticides primarily work against plant diseases. Some examples include: Serenade (Bacillus subtilis strain QST 713), RootShield (Trichoderma harzianum), and Sil-Matrix (potassium silicate). Some of these products contain live microbes that need to stay alive, while others do not (e.g., Sil-Matrix). These biopesticides need to be applied before infection.
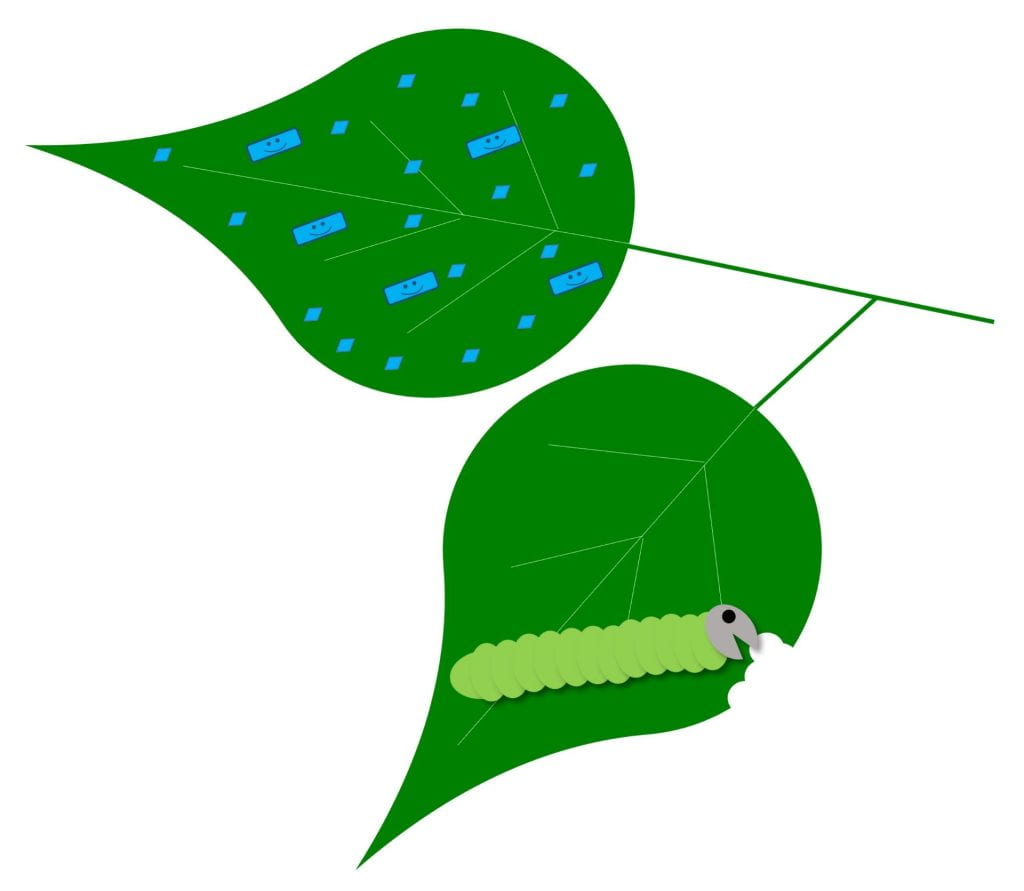
Repel – pest avoids plants treated with biopesticide
Biopesticides with this MOA can work against insect pests, but perhaps only on certain insect life stages. Some products with this MOA could contain live microbes, while others do not. You can evaluate the effectiveness of products with this MOA, not by scouting for dead insects, but by looking for reduced damage or lower insect populations on treated plants. Examples include: Grandevo WDG (Chromobacterium subtsugae strain PRAA4-1 and its spent fermentation products) and products containing azadirachtin.
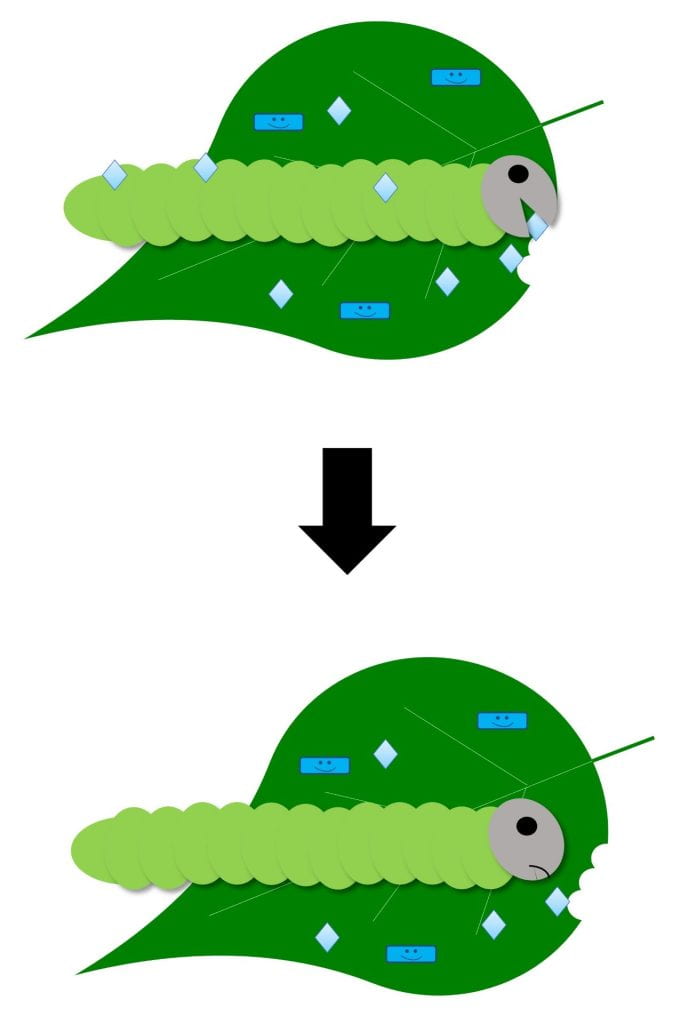
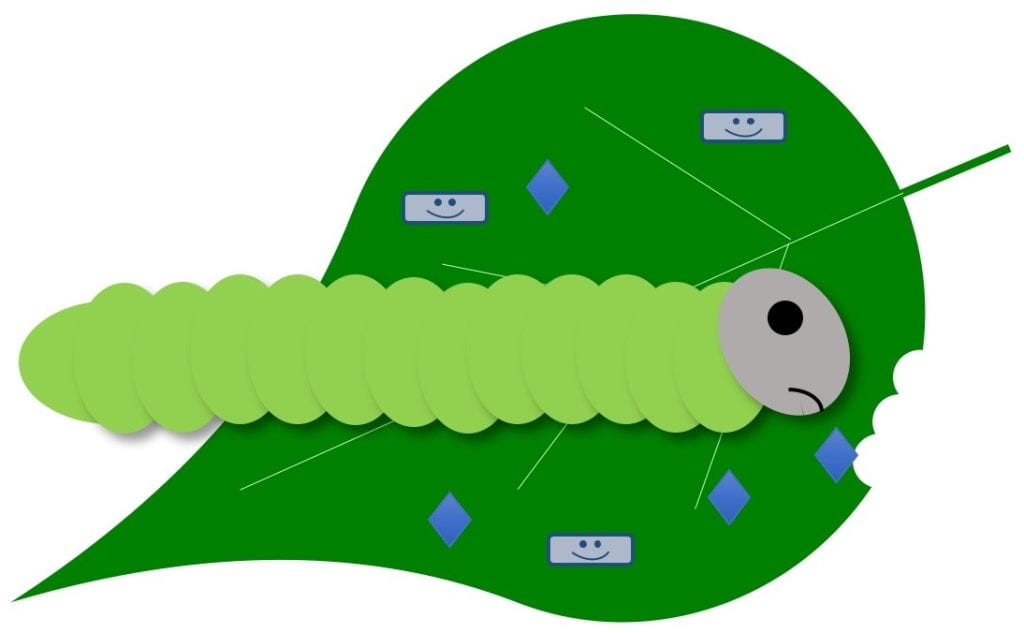
Stop feeding – stops pest from feeding; pest eventually starves
Biopesticides with this MOA can work against insect pests either by contact or ingestion and may only be effective against insects of certain ages or life stages. It depends on the biopesticide and pest. Examples include insect-killing viruses and some types of Bacillus thuringiensis products. Some products with this MOA could contain live microbes, while others do not. Live pests will still be present for some time after applying a product that works in this way, since the pests die of starvation. Watch for feeding damage to stop or a reduction in insect numbers over time to know if the product is working.
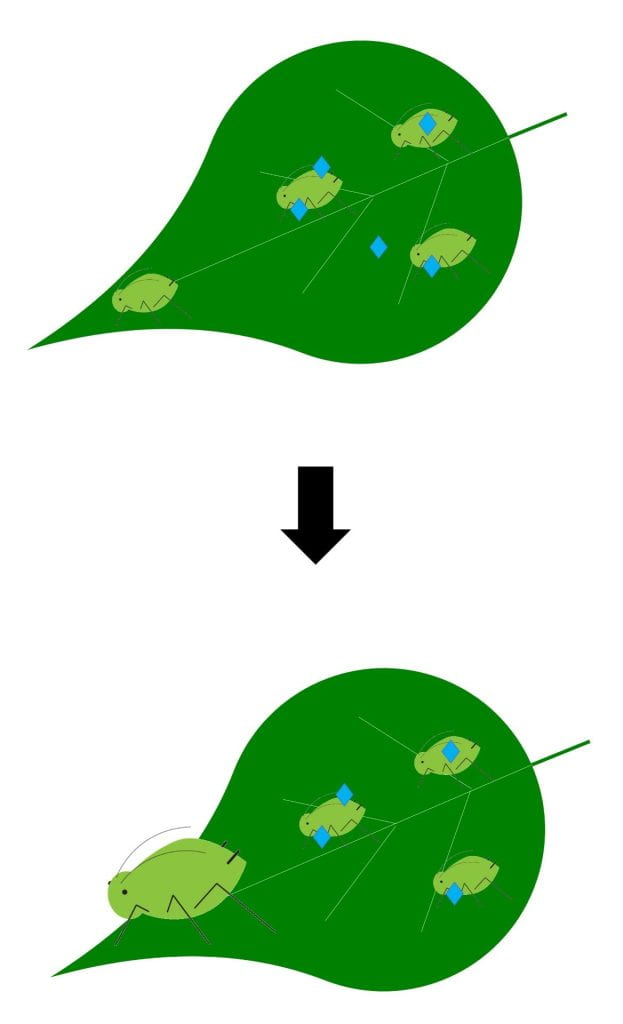
Stop growth – stops pest from growing or molting; pest eventually dies
Biopesticides with this MOA may work against insect pests either by contact or ingestion and may only be effective against pests of certain ages or life stage. It depends on the biopesticide and pest. Examples include Venerate (Burkholderia spp. strain A396) and some products containing azadirachtin. Some products with this MOA could contain live microbes, while others do not. Products with this MOA will not kill pests immediately, but will prevent them from growing or molting. Watch for insect populations to decline over time, but do not expect pests to die immediately.
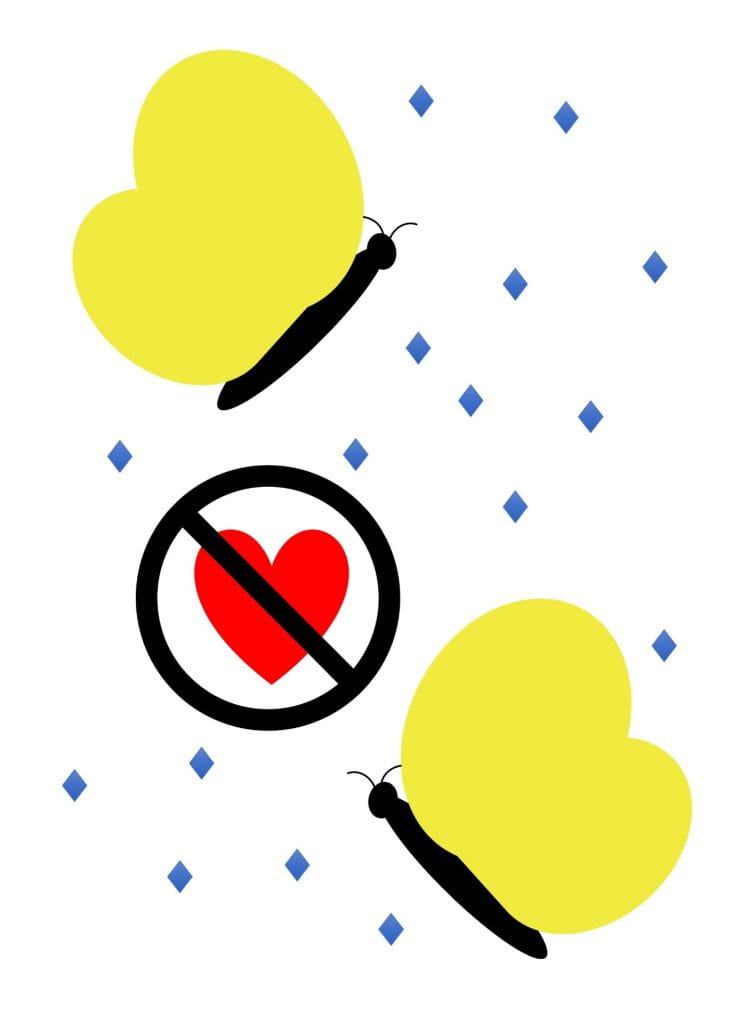
Stop reproduction – hampers pests’ ability to find a mate or produce eggs
The two main groups of biopesticides I know of with this MOA are (1) pheromones that make it hard for male and female insects to find each other, or (2) products that reduce the number of eggs female insects lay. Grandevo (Chromobacterium subtsugae strain PRAA4-1 and spent fermentation products) is an example of the later, but may not work in this way against all ages and species of pests listed on the label. The products I know of with this MOA do not contain live microbes. This mode of action will reduce insect populations in subsequent generations, not the current one. So use it on a pest with multiple generations per season, or in combination with other MOAs.
Things to keep in mind:
If the biopesticide contains live microbes, make sure you…
- store the biopesticide correctly (and for the correct amount of time); check the label.
- confirm compatibility of the biopesticide with other products before tank mixing or applying; read the label and contact the manufacturer with questions.
In addition, if the biopesticide contains microbes that need to stay alive for some period of time after application in order to be effective, make sure you also…
- pay special attention to the recommended optimal environmental conditions for application; start by reading the label.
Remember!
- Biopesticides are pesticides. Their labels are the law. Read the labels and follow them, along with other pesticide application laws in your state.
- Not all biopesticides are permitted for use in certified organic production. Check with your certifier if you have questions.
Questions to ask when you are considering/purchasing a biopesticide
The manufacturer or dealer should be able to tell you:
- How does it work (MOA)?
- Is it alive? Does it need to stay alive to work?
- Special instructions for storage or use? (e.g., temperature, spray tank pH, time of day)
- Is it compatible (in the tank, greenhouse, or field) with other products in use (e.g., pesticides, fertilizers)?
Additional biopesticide Resources
- Summary of modes of action for biopesticide active ingredients
- More information on how to use biocontrol (including biopesticides)
- Summary of biopesticide efficacy results for most crops
- Results from efficacy trials for biopesticides and organic fungicides in vegetables (link will open a dialog asking you to save a spreadsheet)
- Cornell organic vegetable resources (including efficacy results)
This post was written by Amara Dunn, Biocontrol Specialist with the NYSIPM program.