This post was written by Anna Wallis, Kerik Cox, and Mei-Wah Choi (all from Cornell’s School of Integrative Plant Science, section of Plant Pathology and Plant-Microbe Biology). Thanks for sharing your research with us!
Since this is a slightly longer post, here’s a little table of contents:
What products are currently available and where do they fit in?
The verdict on biologicals for fire blight management
Streptomycin is a clear asset in the fire blight arsenal—it is inexpensive, effective, and reliable. However, antibiotics may not always be a viable option. More and more, biological materials are holding their own in the fight, with an increasing number of products on the market claiming protection for both blossom and shoot blight. Biological materials are still relatively new to the apple scene, an industry with a long track record of effective disease management. So why change to biologicals, and how do they work?
There are a multitude of reasons driving the growth of antibiotic alternatives. Organic production eliminated antibiotic use in 2014 in the United States. In European markets, they are prohibited or severely limited. Pressure from regulatory organizations and markets to use more sustainable management techniques will not be slowing any time soon. The prevailing evidence supports that responsible streptomycin applications do not seem to select for resistance in the pathogen. Yet, resistance continues to appear in commercial settings.
So, what are these biological materials and how do they work? In the ‘What is Biocontrol?’ tab above, Amara provides an excellent overview of biocontrol, as defined by the EPA and industry. Here I’ll review the biological modes of action and specific materials available in the context of fire blight management. I’ll also provide a snapshot of how biological programs have performed in our research orchards. There is no intention to endorse any specific trade products, rather this is an attempt to provide a neutral perspective and overview of the current market.
Biological Modes of Action
Biological materials available for fire blight management are typically biopesticides falling into the biochemical or microbial category. This means they are derived from natural sources (i.e. plant extracts or minerals) or they are composed of microcorganisms and/or their products.
To understand how biologicals can be used in fire blight management, it’s first important to review the important features of the disease. A thorough description of the disease cycle, symptoms, and causal organism can be found on this Cornell Fact Sheet. Fire blight is caused by Erwinia amylovora, a bacterial pathogen which preferentially colonizes the floral surface, specifically the stigma or the sticky part of the tip of the female organ. First, enough heat must be accumulated for colonization to occur, which can be predicted by disease forecasting models such as MaryBlyt (if you’re familiar with the disease and pest prediction tool NEWA, this is the model used in the fire blight prediction model there). Then there must be a wetting event to wash the bacteria into the natural openings in the flower, the nectary at the base of the floral cup. Unlike fungi, bacteria cannot penetrate plant cells directly, so they rely on natural openings and tissue damage to invade their host.

Biologicals can disrupt these events by:
- Outcompeting the bacteria during colonization of the plant
- Producing antibiotic metabolites, killing the pathogen prior to infection, or
- Priming natural host defenses, making the plant more resistant to the bacteria. This is called ‘Induced Resistance’
A simplified view of these events is depicted in Figure 2.

Like any product, these materials require precise applications, to ensure they are in the right place at the right time to provide effective control (Figure 3). Materials with competitive action or antimicrobial metabolites that ‘protect’ the flower (protectants) must be applied when the bacteria is present or just before. This enables sufficient, timely colonization or interaction with the pathogen. Induced resistance materials (defense inducers), also called Systemic Acquired Resistance or Induced Systemic Resistance materials (SARs or ISRs), must be applied prior to infection events, with enough time to activate the host response. (Click the image below to enlarge it.)

What products are currently available and where do they fit in?
Blossom protectant type products include both bacteria and fungi. The most well-known examples include: Pantoea agglomerans, a bacterium closely related to the fire blight bacterium and an excellent colonizer of apple flowers, marketed as Bloomtime Biological (Northwest Agricultural Products), and the yeast Aureobasidium pullulans, a fungus, marketed as Blossom Protect (Westbridge Agricultural Products). Another bacterium, Pseudomonas fluorescens, is also an effective competitor and is marketed as BlightBan (NuFarm).
Materials with antimicrobial activity are most often Bacillus species, most commonly strains of B. amyloliquefaciens and B. subtillus. Currently on the market are Serenade Optimum (Bayer), Double Nickel (Certis), and Serifel (BASF).
Products that stimulate Induced Resistance response in the host plant work by stimulating two possible pathways the ISR and SAR, as mentioned earlier. These pathways are related and overlapping in the plant, and scientists are still detangling the complex molecular mechanisms involved in plant protection. Example products include Regalia, an extract of the plant Reynoutria sachaliensis or giant knotweed (Marrone Bio Innovations) and a Bacillus mycoides strain marketed as LifeGard (Certis). Another common product used in induced defense is acibenzolar-S-methyl. This is not a biological, but a synthetically derived product marketed as Actigard (Syngenta).
Many of these products have been recommended as part of an integrative management strategy outlined in an extensive report from The Organic Center, based on results from both research trials and anecdotal experience (Ostenson and Granatstein 2013). Always follow the label on any pesticide (including biopesticides) you use.
Table 1. Biological products for Fire Blight
| Product | Active Ingredient | Mode of Action |
| Firewall | Streptomycin | antibiotic – kills pathogen |
| Blossom Protect | Aureobasidium pullulans strains DSM14940 & 14941 | competitive with pathogen |
| Bloomtime Biological | Pantoea agglomerans strain E325 | competitive with pathogen |
| BlightBan | Pseudomonas fluorescens strain A506 | competitive with pathogen |
| Serenade Optimum | Bacillus amyloliquefaciens strain QST713 | antibiotic metabolites |
| Double Nickel | Bacillus amyloliquefaciens strain D747 | antibiotic metabolites |
| Serifel | Bacillus amyloliquefaciens strain MBI600 | antibiotic metabolites |
| Regalia | extract of Reynoutria (giant knotweed) | resistance inducer |
| LifeGard | Bacillus mycoides isolate J | resistance inducer |
Results from the Cox lab
Our lab conducts extensive trials evaluating efficacy and sustainability of disease management programs in our research orchards at Cornell AgriTech in Geneva. More recently testing has included various biological materials. In these trials, management programs are tested in two orchard blocks: a Gala block and an Ida Red block, established in 2002 and 2004 respectively, both on B.9 rootstock. The trees in these blocks are spaced considerably farther apart than commercial orchards in order to prevent drift between treatments.
Programs targeted either blossom or shoot blight. To provide sufficient disease pressure, trees are inoculated with a high concentration of E. amylovora at bloom. In blossom blight programs, resistance inducers are applied at pink, and protectants are applied at bloom. For shoot blight programs, resistance inducers are applied at petal fall.
Disease pressure varied from season to season, as indicated by the untreated control trees, ranging from 60 to 99 % disease incidence. Across all trials, antibiotics provided the most consistent and reliable control of both blossom and shoot blight, with less than 15% blossom and 5% shoot blight. The biological materials, both protectants applied at bloom and defense inducers applied pre-infection, also provided good disease protection with typically less than 30% incidence depending on the season conditions and the product. Compared to antibiotic programs, these materials showed greater variation both within and between seasons (i.e. greater standard deviation within a treatment and different top performers in different seasons). In seasons with lower disease pressure, biological programs tended to perform as well as antibiotics. Some of the specific results from 2015-17 are shown in Figure 4 (click the image to enlarge the graphs).
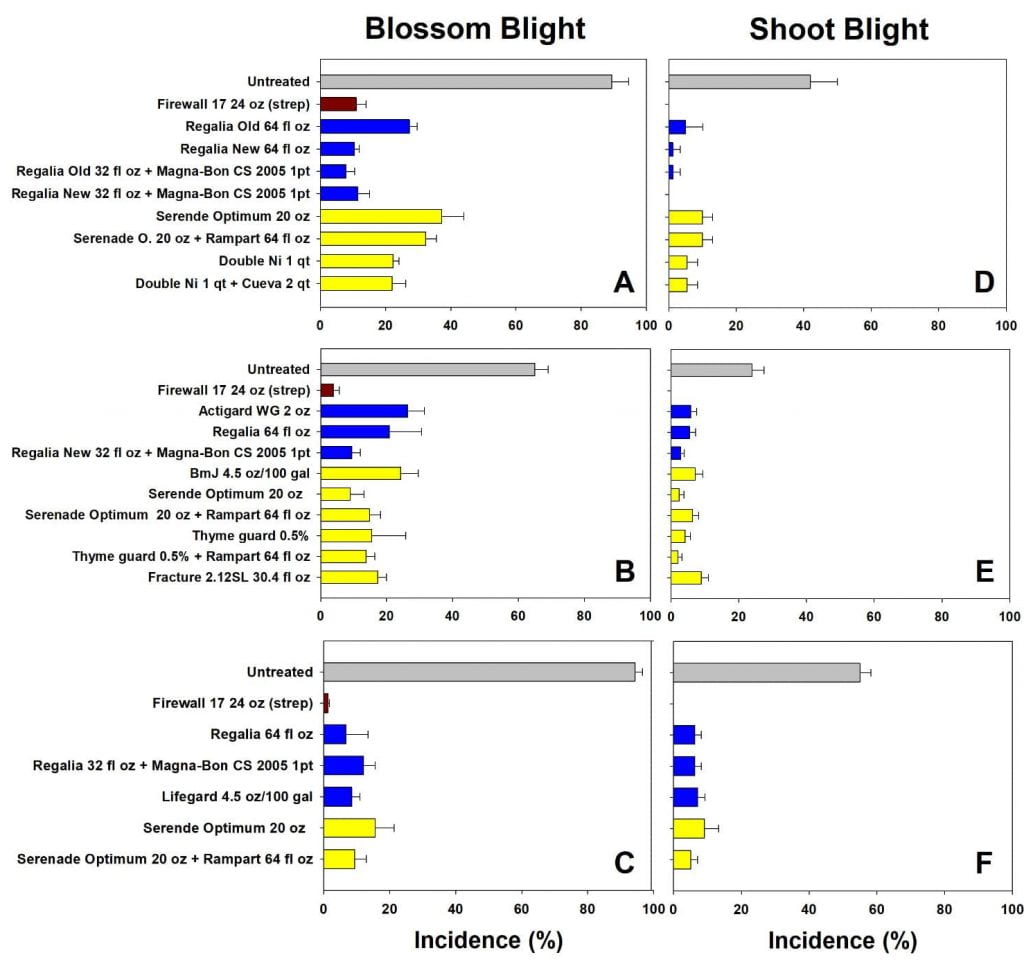
The verdict on biologicals for fire blight management
Do we recommend biological materials for fire blight management? Overall, the answer is generally yes. There are several important considerations to consider. In our research orchards, the system is challenged with a very high level of inoculum to examine fine differences in product performance. These inoculum levels are much higher than would be present in most commercial orchards. Hence, we expect all programs would perform even better in a commercial setting. In addition, combinations of products seem to be the best: for example, pairing a defense inducer applied at bloom with a protectant material at bloom to control blossom blight, with follow up defense inducer applications for shoot blight. We also expect efficacy of biological materials to improve in the future. Changes in formulations improving activity (note the old and new Regalia formulations in Figure 3), as well as shelf life, tank mixing, and storage happen fairly regularly and will make products more accessible and affordable for growers.
Biologicals are still relatively new materials. As with any product, there is still much to learn about how products work in the field, the most effective management programs, and translating best practices from research to commercial settings. We believe they are a valuable part of an integrated fire blight management approach, including good cultural and mechanical practices such as planting resistant cultivars and rootstocks and removing inoculum from the orchard.
You can learn more from these sources:
Ostenson, H., and Granatstein, D. Grower Lessons and Emerging Research for Developing an Integrated Non-Antibiotic Fire Blight Control Program in Organic Fruit. The Organic Center. November 2013. Available at: https://www.organic-center.org/wp-content/uploads/2013/07/TOC_Report_Blight_2b.pdf
Pal, K., and Gardener, B. 2011. Biological Control of Plant Pathogens. The Plant Health Instructor, APS. Available at: https://www.apsnet.org/edcenter/advanced/topics/Pages/BiologicalControl.aspx.
Turechek, W. W., and Biggs, A. R. 2015. Maryblyt v. 7.1 for Windows: An Improved Fire Blight Forecasting Program for Apples and Pears. Plant Health Progress. 16:16–22. Available at: https://www.plantmanagementnetwork.org/pub/php/volume16/number1/PHP-RS-14-0046.pdf
