Why grow perennial wildflowers?
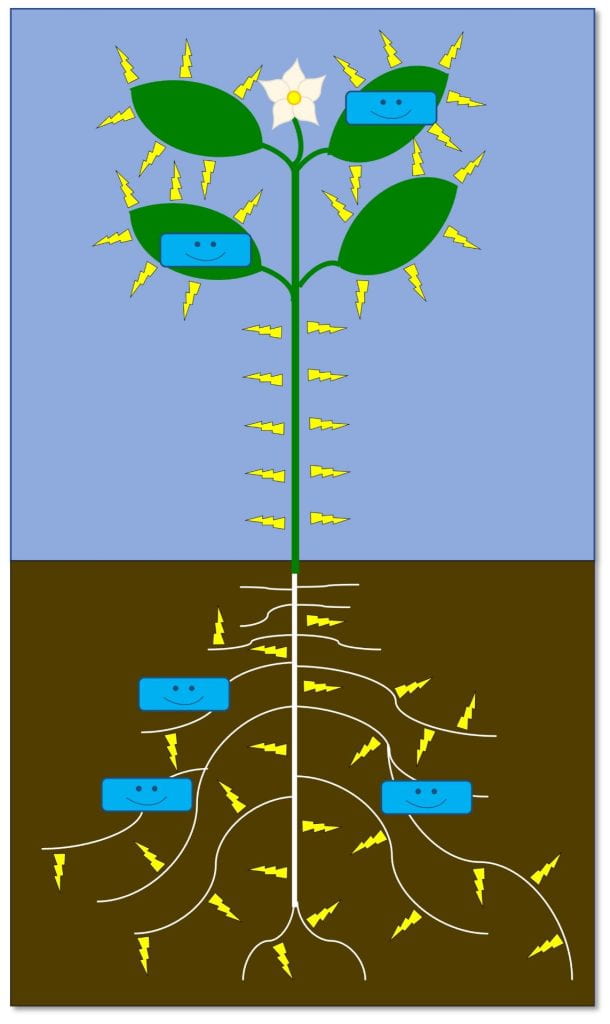
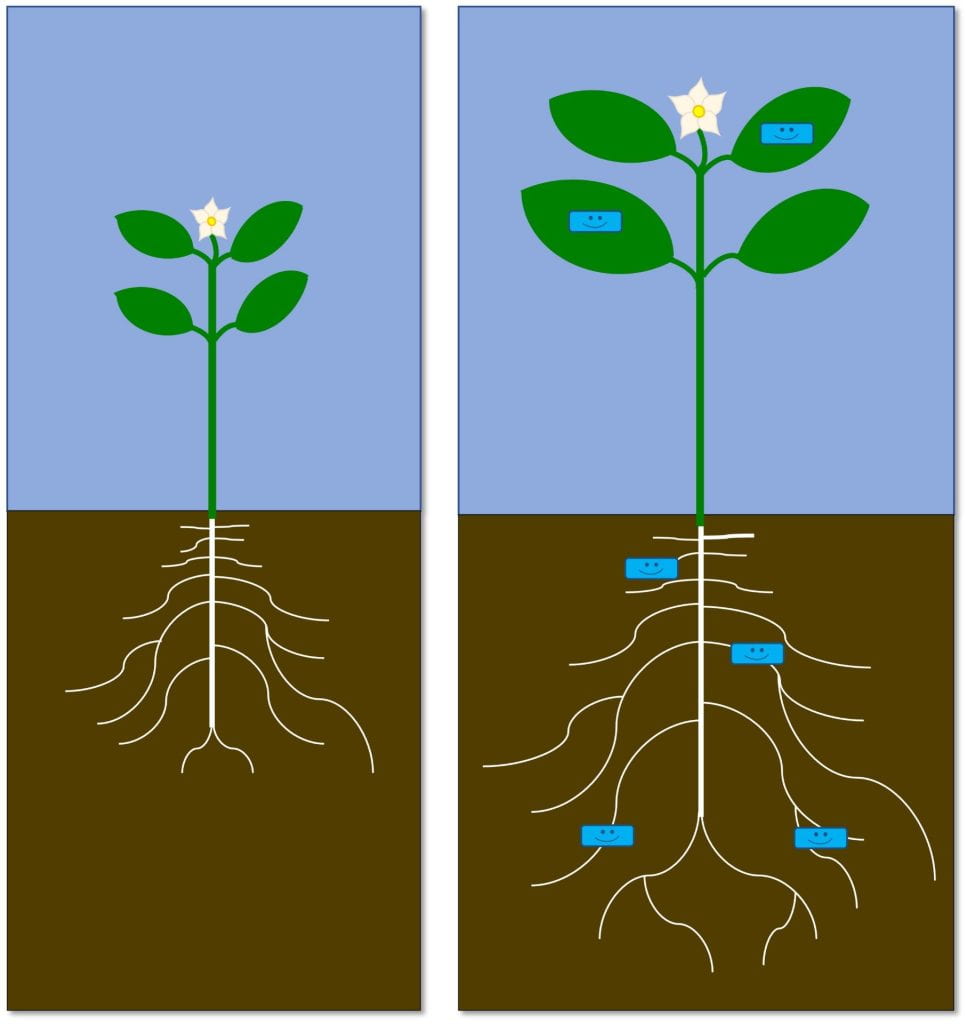
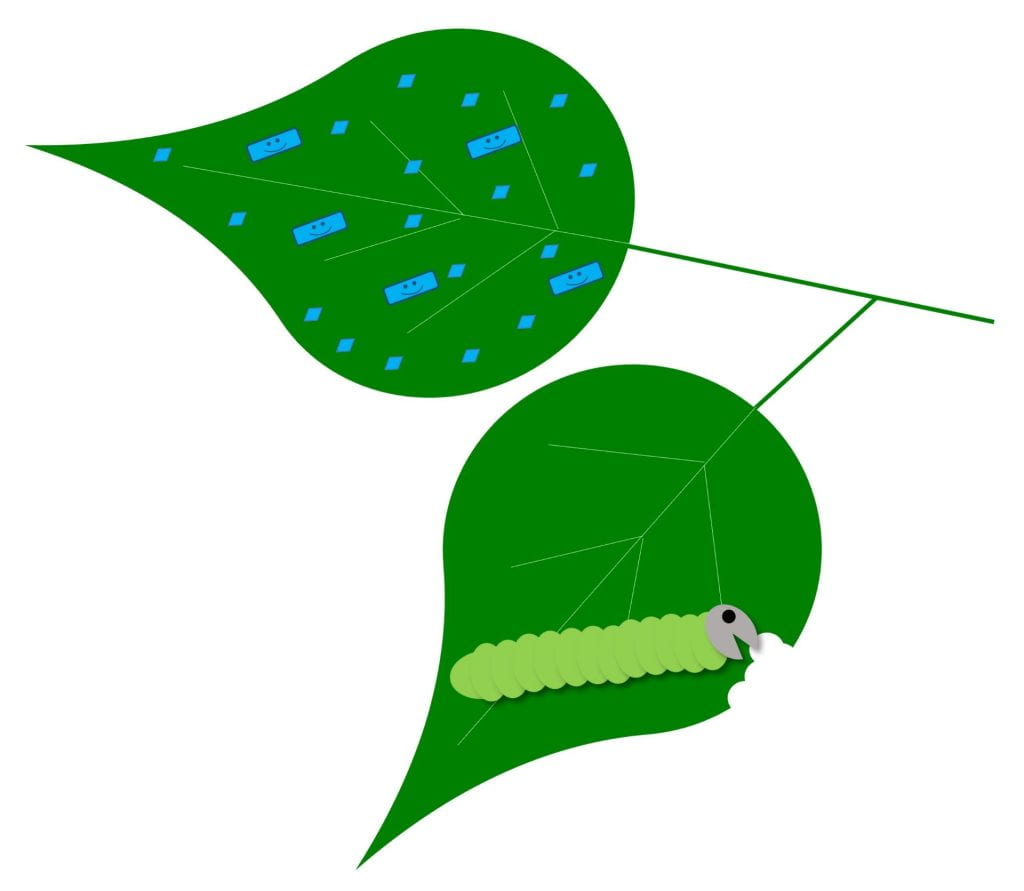
Native wildflowers feed and shelter beneficial insects that pollinate our plants and help us control pests. Beneficial insects include a diverse collection of pollinators — not just honey bees — as well as natural enemies (flies, wasps, beetles, bugs and other insects that kill pests). While not technically insects, spiders and predatory mites also serve as natural enemies of pests. Cooperating with insect natural enemies for pest control helps us reduce damage from pests while reducing risks to the environment and people from other pest management strategies like pesticides.
The pollen and nectar produced by these native flowers – and many non-native flowers – either directly feed these friendly insects, or feed other insects that are prey for natural enemies. The leaves and stems also provide shelter for beneficial insects year-round. While we’re focusing on flowers today, many grasses also provide this shelter. Beyond all the ecological benefits, native wildflowers are beautiful!
Preparing perennial seeds to germinate

In this part of the world (now known as upstate New York), seeds from native perennial wildflowers that mature in summer and fall experience a lot of “weathering” between maturation and germination the following spring. This includes cycles of freezing and thawing temperatures as well as varying levels of moisture during the winter. They may even pass through the digestive system of animals. This is why planting perennial wildflower seed in the fall is a great option. You are allowing nature to do the work of preparing the seeds for germination in the spring. If you harvest seeds of native perennial wildflowers and want to start them in the spring with other seeds, you will need to do this work yourself.
Some seeds have very hard seed coats that need to be broken by physical force (for example scratching with sandpaper, a file, or a knife), heat (for example, submerging in boiling water), cold (for example, freezing), or chemicals (like a strong acid). This is called seed scarification. Seeds that require scarification – and those that don’t – may also require stratification, which signals to the seeds that they can “wake up” (called breaking dormancy) and start germinating. Seed stratification generally requires exposing the seeds to the right level of moisture and the right temperature for the right length of time. Some examples include:
- Hot water soak – Pour hot water over seeds and allow them to soak overnight or for 24 hours
- Cold moist stratification – Mix seeds with sterile sand, vermiculite, or another substance that won’t hold too much moisture. You could also place seeds between layers of a damp paper towel or coffee filter. Make sure that whatever medium you use is only damp and not too wet. Put the mixture in a plastic bag or closed container in your refrigerator for the required amount of time.
If you buy perennial wildflower seeds, they may have already been stratified. Follow the instructions that come with your seed or reach out to the seller for more information.

Species-specific instructions
Examples of perennial wildflower seeds that benefit from scarification and/or stratification follow. After these seeds are scarified and/or stratified, germination may be improved by putting them in a warm place (but not for all species). Remember that you can always sprinkle seeds on well-prepared, weed-free soil outside in the fall and let the winter do the work for you! Also, your experience germinating these seeds may differ from the recommendations below. There is so much beautiful diversity in these perennial wildflowers!
- Blue false indigo (Baptisia australis) – First scarify the seeds. Gently rub them between medium-grit sandpaper to scratch the seed coat. Then use the cold moist stratification method, leaving them in the refrigerator for about 10 days.
- New York Ironweed (Vernonia noveboracensis) – Use cold moist stratification, keeping seeds chilled for 60 days.
- Asters (Symphyotrichum spp.) – If you look up how to seed asters, you’ll see different instructions for different species, including seeding them in the spring without stratification and using a 60-day cold stratification period. Amara tried germinating Symphyotrichum oblongifolium (aromatic aster) seed and had better luck with cold moist stratification than seeding without stratification. You might see different results with different species.
- Milkweed (Asclepias spp.) – Store (well-dried!) seeds in the freezer for several months (e.g., from the time you harvest them in the fall, until you are ready to start them in the spring). Learn more from Petra at Fruition Seeds.
- Coreopsis (Coreopsis spp.) – Note that some coreopsis species are annuals in upstate NY. Perennial species will likely germinate best with cold moist stratification (30-60 days, depending on species).
- Echinacea (Echinacea purpurea) – These seeds will likely germinate just fine if you plant them in moist soil and provide warmth (e.g., a heat mat) just as you would annual seeds.
- Blazing star (Liatris spicata) – Moist stratify for 60 days before planting seeds.
- Beard tongue (Penstemon spp.) – Cold stratify for 30-60 days before planting seeds. Some species may require light for germination. Cover seed of these species with only a very thin layer of potting mix (if any) and provide light when germinating.
- Mountain mints (Pycnanthemum spp.) – You should be able to germinate seeds without stratification, but they will likely germinate better with light. Cover seed with only a very thin layer of potting mix (if any) and provide light when germinating.
- Rudbeckia (Rudbeckia spp.) – Different species of Rudbeckia may require different stratification (or none) for seed to germinate. For many species, cold moist stratification for at least 30 days is recommended.
- Goldenrod (Solidago spp.) – There are many species. Most will likely germinate best if seed is moist stratified for 60 days, and then receive light after seeding. Cover seed with only a very thin layer of potting mix (if any) and provide light when germinating.
- Spiderwort (Tradescantia ohiensis) – For this species, you are better off direct-seeding in the fall. The recommended cold moist stratification period is 120 days, and these seeds will germinate better in cool soil.
- Golden alexanders (Zizia aurea) – You may have better luck sowing these seeds outside in the fall than trying to start them inside in the spring. Their leaves are fairly distinctive (like celery) so seedlings are easier to spot outdoors. If you do want to try starting them indoors, use a 60-day cold moist stratification.
For some of the species that require longer stratification, you may be worried that you’re running out of time this spring. Remember that even after you have prepared your seeds to germinate, they will likely need around 6 weeks to germinate and grow big enough to consider transplanting outdoors. Don’t despair! Fall is a great time to transplant perennial wildflowers. And perhaps your heat mats and grow lights (or other seed germination spot) are otherwise occupied starting annual vegetable and herb transplants this spring. If you start stratifying seeds in early May, even those that require 60 days in the fridge will be ready for germination in early July. Then your seedlings will have plenty of time to grow big and strong before fall transplanting. Just be sure to keep them watered.
Resources consulted on seed germination:
- Growing Native Plants from Seed. North Carolina Botanical Garden.
- How to Germinate Native Seeds. Prairie Moon Nursery.
- Page-Mann, Petra. How to Harvest Milkweed Seeds. Fruition Seeds.
This post was written by Amara Dunn-Silver, Biocontrol Specialist and Elizabeth Lamb, Ornamentals IPM Coordinator with the NYSIPM Program.
This work is supported by NYS Departments of Environmental Conservation and Agriculture and Markets.