Gary C. Bergstrom
School of Integrative Plant Science, Plant Pathology and Plant-Microbe Biology Section, Cornell University, Ithaca, NY 14853
Printer-friendly .pdf version.
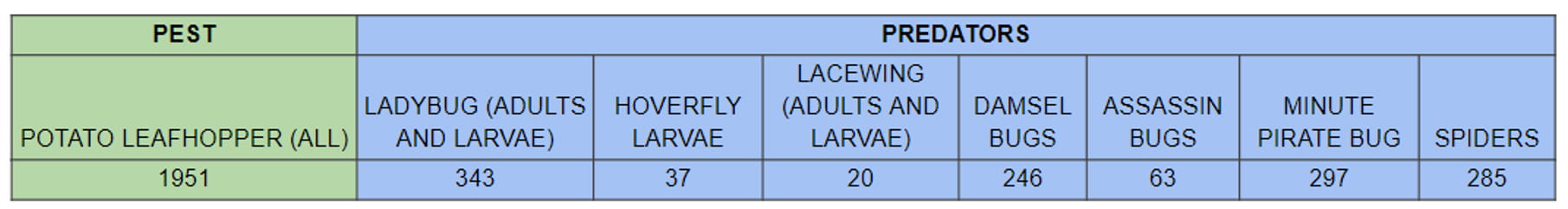
The presence of the corn stunt spiroplasma was confirmed in corn fields in four non-contiguous New York Counties (Erie, Jefferson, Monroe, and Yates) in October 2024. The causal agent of corn stunt, Spiroplasma kunkelii, belongs to a specialized class of bacteria known as mollicutes which also includes phytoplasmas. Spiroplasma cells lack walls, and they have a short, spiral shape. They live an obligate lifestyle, i.e., they survive and reproduce only in living leafhopper hosts and in the phloem sieve elements of specific plant hosts. The pathogen that causes corn stunt is transmitted by the corn leafhopper, Dalbulus maidis, also not documented previously in New York (Figure 1). That status changed this October as individuals of D. maidis were caught on a yellow sticky trap in Jefferson County. One captured leafhopper was confirmed by molecular tests to be infected by S. kunkelii. This is the first documentation of the corn leafhopper and of S. kunkelii in both corn leaves and corn leafhoppers in New York.

How is the spiroplasma transmitted and spread?
The corn leafhopper, D. maidis, can acquire spiroplasma through its probing mouthparts in less than an hour of feeding in phloem tissues of infected corn plants, but it can take up to two weeks of spiroplasma replication in the leafhopper’s body before the insect can then transmit the spiroplasma into the phloem of healthy corn plants. Symptoms don’t generally appear until about a month after plants have been infected. The most severe symptoms are the result of infection at early corn growth stages (from VE to V8). An infected leafhopper can transmit spiroplasma to many nearby plants and can also be blown by air currents and deposited into distant corn fields.
Where did the leafhopper and spiroplasma in New York come from?
Corn stunt is a disease complex first described nearly 80 years ago in the Rio Grande Valley of Texas. Spiroplasma kunkelii is the principal pathogen causing corn stunt. However, other pathogens, either alone or in combination, also can cause corn stunt; these pathogens include the maize bushy stunt phytoplasma, the maize rayado fino virus, and the maize striate mosaic virus. Leaf samples from New York have been archived for later testing for these additional pathogens. Over past decades, there have been observations of corn stunt symptoms in several southern and eastern states but epidemics of corn stunt with well documented isolation of S. kunkelii have been primarily in Texas, Florida, and California. In recent years, corn stunt has occurred as a yield-reducing disease primarily in Mexico, Central and South America, particularly in Argentina and Brazil. The principal vector, the corn leafhopper, can be transported long distances by air currents and carries the pathogen within it. While there is no direct proof, it is very likely that long-distance atmospheric transport of the corn leafhopper into the Midwest and Northeast in 2024 was aided by storm systems that moved north from southern states.
What are the symptoms of corn stunt?
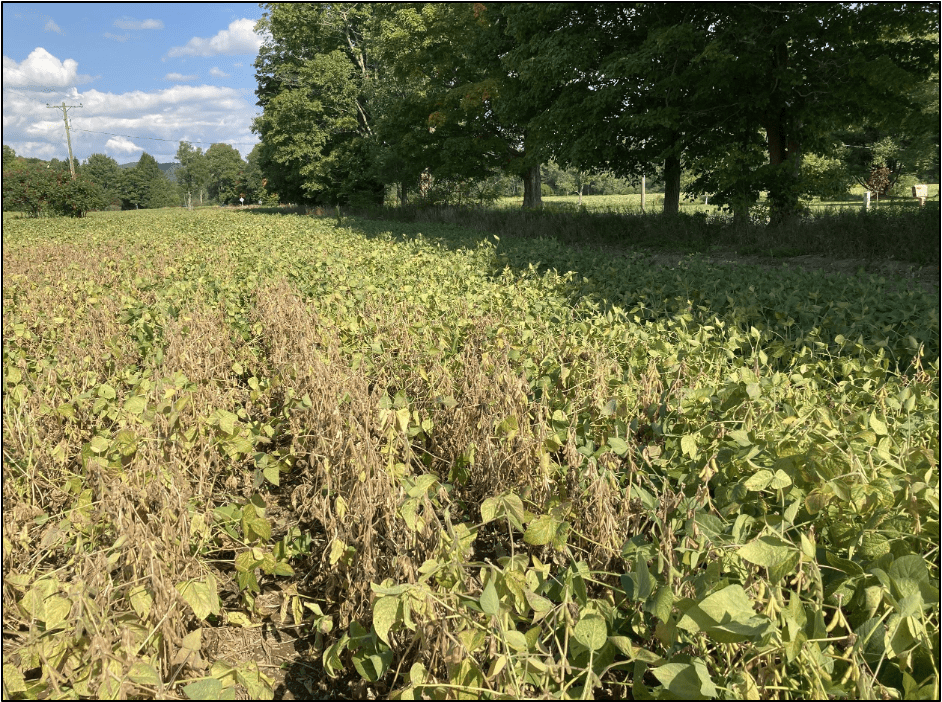
Corn stunt symptoms present similarly to other stresses in corn, including drought, soil compaction, and phosphorous deficiency. Leaf blades and sheathes can show white or yellow stripes (loss of chlorophyl) or red or purple streaks (anthocyanin pigments) and plants may show premature senescence (but without stalk rot) (Figure 2). Corn stunt varies from several common stressors in that plants can show significant stunting and ear abnormalities such as poorly filled ears, no ears or multiple ears at the same node. Symptoms may appear in patches within a field or across larger portions of a field.

How was corn stunt detected in New York?
From conference calls with my field crop pathology counterparts in southern and corn belt states this summer, I became aware that, in association with stunted and discolored corn plants, corn stunt and corn leafhopper were being observed further north of their usual ranges in 2024. Yet, I thought that New York was at a sufficiently northern latitude to avoid these problems. I credit a very observant agronomy specialist, Rafaela Aguiar with Kreher Family Farms, for noticing unusual symptoms in field corn in Erie County in late summer. Rafaela, a native of Brazil and with previous agronomic experience in South America, thought the symptoms resembled corn stunt which she had seen in South America. Though I was skeptical, it turned out that Rafaela was correct. We initially collected samples of symptomatic plants (Figure 2A) from three Erie County fields and sent them to the Diagnostic Lab at Oklahoma State University. Two of the three fields came back as strongly positive for the corn stunt spiroplasma. In a race against corn harvest and frost, samples were then collected from corn in other counties where similar symptoms had been reported. Samples from Jefferson, Monroe, and Yates Counties were also positive (Figure 2B). I suggest that, given more time for scouting in October, corn stunt may have been diagnosed in many more corn fields in New York this year.
What does this mean for future corn production in New York?
Documentation of the pathogen and its insect vector in New York in 2024 demonstrated that corn stunt could occur in New York in future growing seasons. And if spiroplasma-infected corn leafhoppers arrive at earlier corn growth stages, significant yield losses could result. Then again, the atmospheric pathways that carried corn leafhoppers to New York in 2024 might not be repeated for several years. Many presume that the corn leafhopper will not overwinter as far north as New York, but, with climate change, that may be proven incorrect. There is much that we don’t know. Cornell University, Cornell Cooperative Extension, and the New York State Integrated Pest Management Program have committed to participate in a Corn Stunt Working Group of plant pathologists and entomologists in states affected by corn stunt and corn leafhopper. One aim of the group is to deploy a common protocol to monitor the corn leafhopper during the 2025 growing season. Also, the Cornell Plant Disease Diagnostic Clinic is gearing up to offer a molecular test for corn stunt spiroplasma in 2025.
How will the corn stunt disease complex be managed?
Awareness and accurate diagnosis of corn stunt and regional monitoring for corn leafhopper are necessary first steps in managing this complex. Based on limited observations in 2024, it appears that corn stunt could cause significant yield reductions under New York corn growing conditions. Plant breeding is the long-term solution to prevent corn yield losses. Hybrids with moderate resistance to the spiroplasma and / or the leafhopper have been deployed in Latin American countries to manage the corn stunt complex. International companies that sell seed in the U.S. as well as Latin America are aware of which germplasms are most promising for incorporation into hybrids for northern temperate areas such as ours. I do not expect much choice of resistance in northern hybrids in 2025. Management of corn leafhopper populations with insecticides at corn vegetative stages to reduce corn stunt deserves further investigation. My principal advice to New York growers in 2025 is to plant corn at the earliest recommended date to avoid arrival of leafhoppers at the most vulnerable plant stages for infection by spiroplasma.
Acknowledgements:
I gratefully acknowledge agronomist Rafaela Aguiar of Kreher Family Farms for her keen observation of corn stunt symptoms and her continuing cooperation. Colleagues Michael Stanyard (Cornell Cooperative Extension Northwest New York Dairy, Livestock, and Field Crops Program) and Michael Hunter (New York State Integrated Pest Management Program) were instrumental in collecting corn leaf samples and leafhoppers from additional sites in New York. Identification of corn leafhopper and corn stunt spiroplasma would not have been possible without the expert help of colleagues at Oklahoma State University including professors Maira Duffeck and Ashleigh Faris, and diagnostician Jennifer Olson.
References:
Faris, A.M. and M. Duffeck. 2024. Corn leafhopper leads to corn stunt disease across Oklahoma – August 12, 2024. Oklahoma State University Extension News, EPP23-17.
Klaudt, J. 2004. Corn leafhoppers carrying corn stunt make first-time appearance in Kansas. Kansas State University Research and Extension News Release – October 16, 2024.
Redinbaugh, M.G. 2016. Diseases caused by mollicutes. Pages 16-19 in: Compendium of Corn Diseases (Fourth Edition), ed. G.P. Munkvold and D.G. White. APS Press, St. Paul, MN.