Author Archives: Jonathan Gorman
Lunch Lecture: Overview of the Cornell Wildlife Health lab
Dinner Lecture – The Asian Vulture Crisis: Diclofenac and its profound impact on a keystone species
Lecture: Conservation in Uganda
Dinner Event: Careers in Wildlife Conservation, Zoo, and Exotic Medicine
What Wildlife Workers Should Know About Avian Anatomy
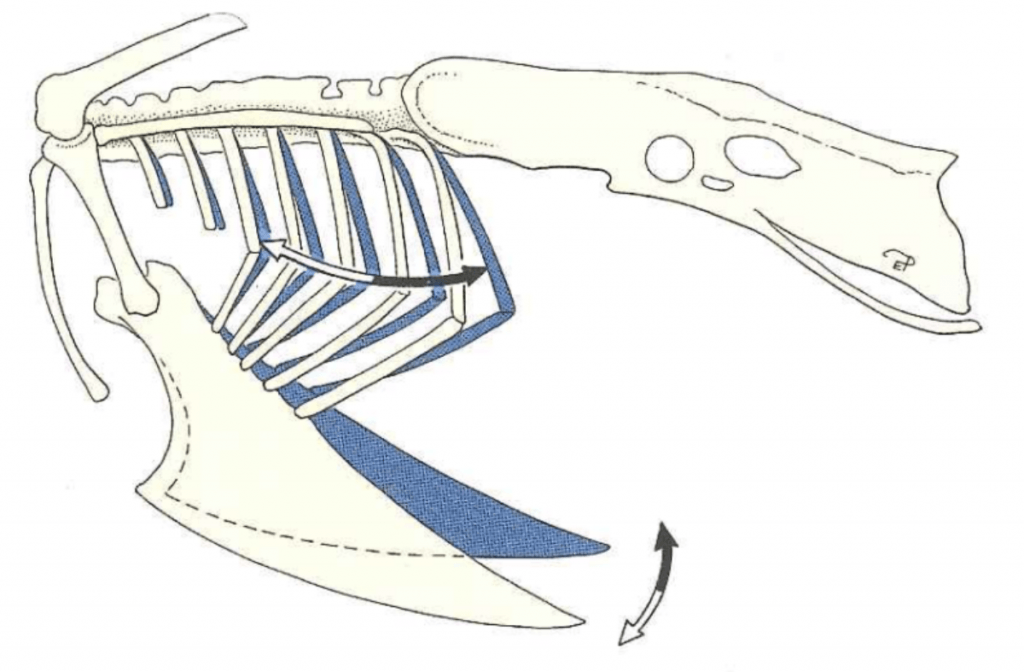
Figure 1: Motion of the keel and ribs during inspiration (white arrows) and expiration (black arrows). Credit: König et al.
As veterinary students, virtually all the anatomy that we learn in the core curriculum is mammalian anatomy, but most of the patients coming into the Janet Swanson Wildlife Health Center are birds. To help fellow veterinary students and other wildlife workers, I have created this post as a brief guide to avian anatomy, focusing specifically on aspects which are directly relevant to providing routine avian care at a wildlife hospital.

Figure 3: Example of a good hold for a raptor. Notice how, like the blue jay, this great horned owl is restrained from the back and by the feet. Credit: Jonathan Gorman
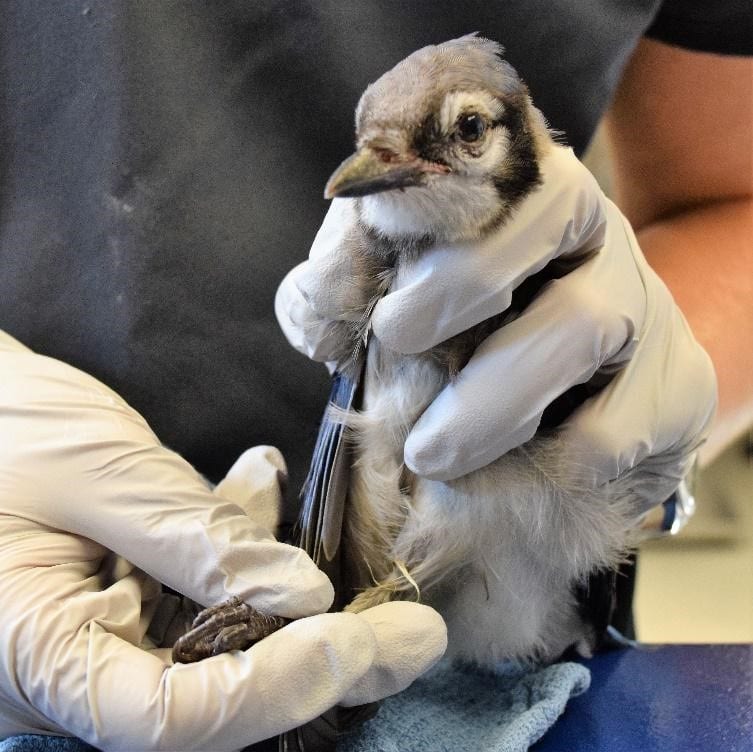
Figure 2: Example of a good hold for a passerine. Notice that this blue jay is primarily restrained from the back and by the legs so as not to put pressure on the sternum. Credit: Jonathan Gorman
Respiratory Anatomy
In birds, the sternum exists as a large fused structure called the keel. Unlike mammals, birds do not have a diaphragm; during inspiration they rely on the external intercostal and levator costarum muscles which move the ribs laterally and the sternum cranio-ventrally (Figure 1). When handling birds, it is very important not to put pressure on the keel since this prevents these movements and can potentially cause asphyxiation. It is therefore necessary to use appropriate restraining methods that don’t place pressure on the keel (Figures 2 and 3). During inspiration, the weight of the viscera helps to pull the sternum in the cranio-ventral direction. However, when a bird is flipped on their back, the viscera weighs the sternum down making inspiration more difficult. For this reason, extra caution must be taken when placing birds on their backs for long periods of time.
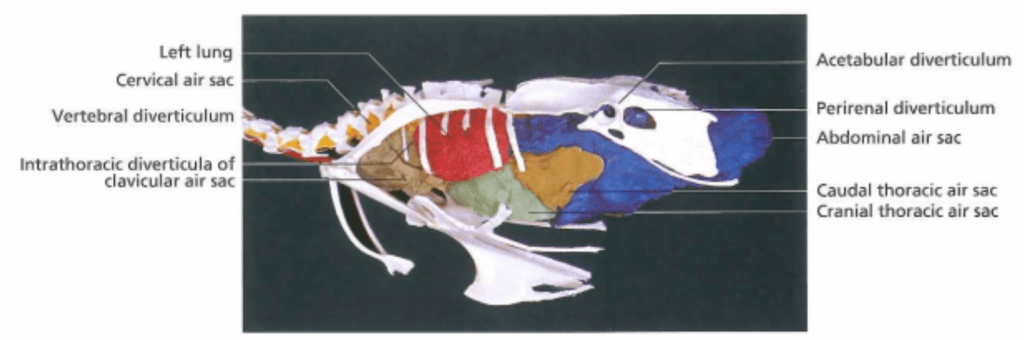
In the mammalian respiratory system, air enters the lungs during inspiration and exits the lungs during expiration in a bidirectional manner . Birds instead have a unidirectional system of air flow that relies on thin-walled cavities attached to the lungs called air sacs. This allows fresh air to pass through the lungs during both inspiration and expiration (Figure 4). The air sacs extend throughout most of a bird’s body cavity (Figure 5), covering the viscera, and even projecting into major bones. This is important to keep in mind during subcutaneous injections, so that one does not inject into an air sac. Pulling back on the plunger to check for negative pressure before giving a subcutaneous injection will ensure that an air sac has not been penetrated.
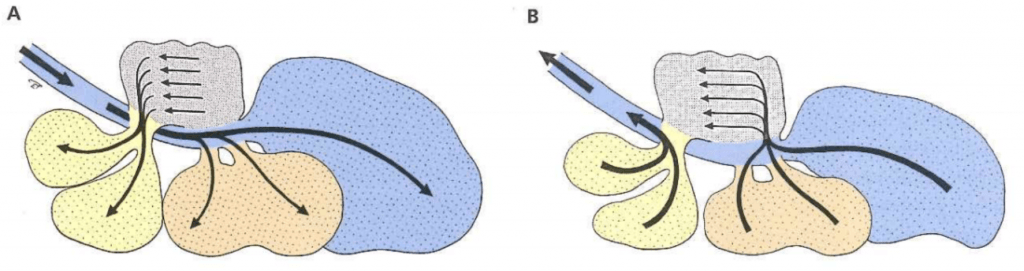
Figure 4: Schematic of avian respiration. (A) During inspiration, air in the trachea flows to the caudal air sacs (caudal thoracic and abdominal air sacs) while air in the lungs flows to the cranial air sacs (cranial thoracic and clavicular air sacs). (B) During expiration, the air in the caudal air sacs flows through the lungs while the air in the cranial airs sacs flows out the trachea. Credit: König et al.
Subcutaneous Injections
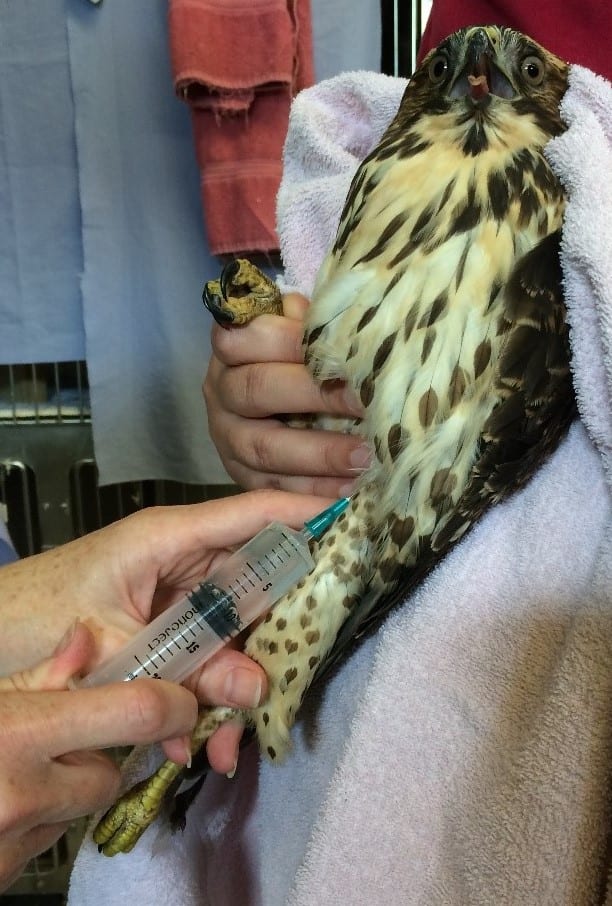
For birds, there are two main sites of subcutaneous injection used at the Wildlife Health Center. The preferred site for subcutaneous fluid administration is the inguinal fold, which is located at the proximal aspect of the femur (Figure 6). Another site used for injections in large birds is the interscapular region, which is over the back and between the shoulders. If injecting here, it is important not to get too close to the neck, since this would risk puncturing the cervical air sac.
Gastrointestinal Anatomy
When giving food or medications to a bird by gavage (i.e. using a tube or crop needle that goes down the throat), it is very important to place the instrument down the esophagus and not the trachea. The esophagus in birds has a very broad opening at the back of the mouth. In contrast, the glottis (i.e. tracheal opening) appears as a slit on the floor of the mouth just caudal to the tongue (Figure 7). In order to avoid aspiration of fluid when giving liquid medication, the medicine should be administered where it will not enter the glottis, either behind the glottis or at the tip of the beak where it will flow to the sides of the mouth.
Unlike in mammals, the avian esophagus runs down the right side of the neck. When inserting a tube or crop needle, correct instrument placement can be verified by palpating the instrument through the esophagus to make sure it is not in the trachea – if it is in the correct place, one should feel two tubes: the gavage tube or crop needle in the esophagus, and the trachea. If only one tube can be felt, the instrument may be inside the trachea. Additionally, if the instrument is placed correctly one should be able to see that the opening of the glottis is clear (Figure 8).
Some species of birds have an enlargement of the esophagus at the level of the thoracic inlet called a crop. The crop temporarily stores ingesta before it enters the proventriculus (i.e. the glandular stomach). When using a tube or crop needle to gavage a bird, the tip of the instrument should be all the way in the crop to avoid aspiration. It is therefore a good idea to take note of how much length is needed to reach the thoracic inlet so that the instrument is placed far enough down the esophagus.
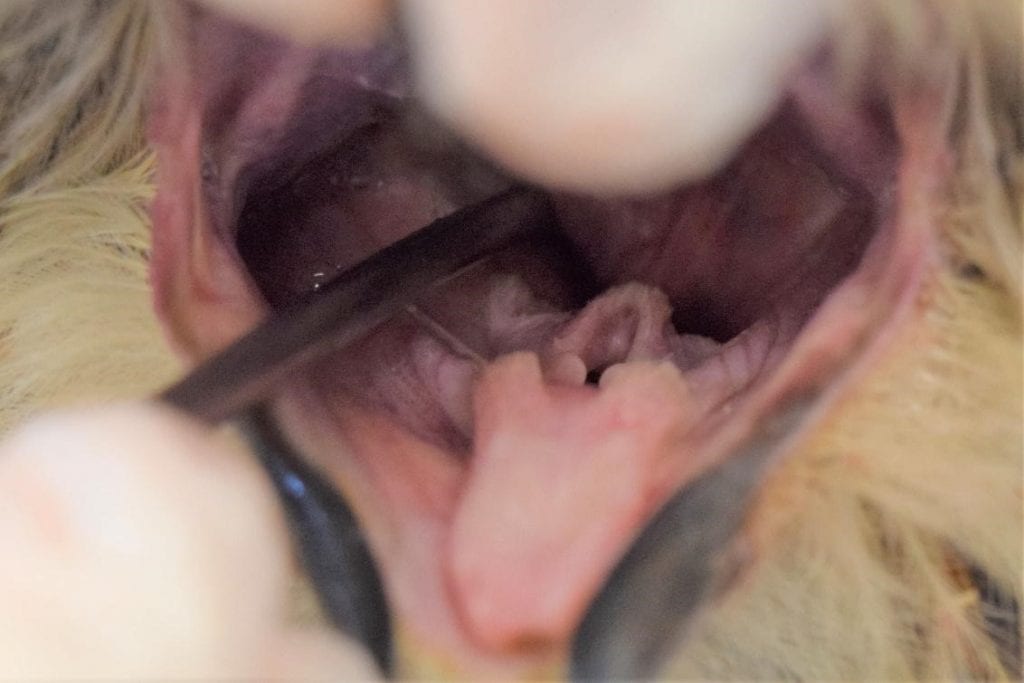
Figure 8: Correct placement of a crop needle for a great horned owl. The needle is entering the esophagus and the glottis is clear. Credit: Jonathan Gorman
Anatomy of Elimination
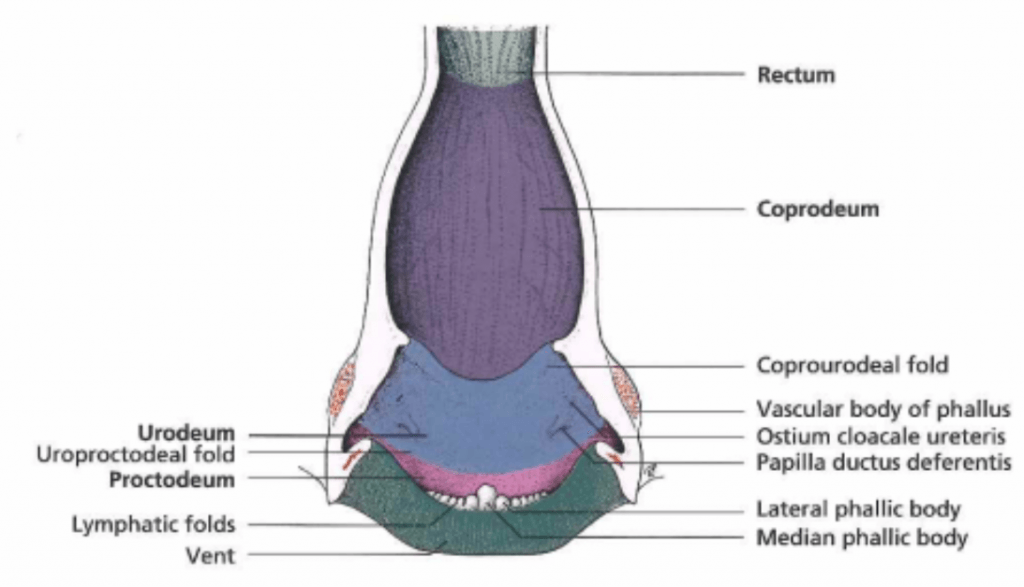
The final part of the gastrointestinal tract in birds, the cloaca, is divided into three parts: the coprodeum, the urodeum, and the proctodeum (Figure 9). Gastrointestinal contents enter the coprodeum from the rectum. They then pass through the urodeum where the urinary and reproductive tracts open. The proctodeum is the final segment of the cloaca, and it houses the copulatory organ (phallus) in males, something important to note when sexing some species of birds.
Because the ureters open into the cloaca, birds pass their urine with their feces; this is important to note when evaluating feces and urates. For instance, a bird with excessively watery excreta may have diarrhea or it may have polyuria. Diarrhea would present as liquid feces while polyuria would present as an excess of liquid surrounding a relatively firm fecal mass. One must take into account that the normal appearance of feces and urates varies significantly by species and diet (Figure 10).

Figure 10: Different images of ‘normal’ feces and urates from different types of birds. The one on the left from a pigeon is typical of granivores; the feces is firm and easily distinguished from the urates which are thick and pasty. The middle image from a great horned owl is typical of raptors which have thick or thin viscous feces mixed with urine. ‘Soft-feeders’, namely insectivores and frugivores, produce feces and urates like that presented on the right from a blue jay. The feces is thin but the color and consistency can vary greatly depending on diet. Credit: Jonathan Gorman
Integument System
The beak is covered in a hard keratinized sheath, the rhamphotheca. Most birds have an aggregation of sensory receptors at the tip of the beak known as the bill tip organ. This organ is especially well-developed in ducks and geese but is absent in pigeons and sparrows. It is important to note when working around the beak that this can be a sensitive area.
While birds do not have sweat glands, some of them do have an important gland located above the tail called the uropygial gland. The uropygial gland produces an oily secretion which certain birds, particularly waterfowl, spread around over their feathers during preening to create a waterproof film. This waterproof film can be degraded by urates, which is why it is important to keep housing for waterfowl clean. This is especially important for birds that can no longer preen themselves due to injury.
Acknowledgements
I would like to thank Dr. Childs-Sanford for providing feedback and advice on this post. I would also like to thank Isabel Jimenez (’19) for helping me come up with ideas and for sharing her knowledge on wildlife care. Lastly, thank you to the Janet L. Swanson Wildlife Health Center, its staff, and its volunteers, for providing me with the inspiration and photo opportunities for this post.
References
König, Horst E, Rüdiger Korbel, Hans-Georg Liebich, Hermann Bragulla, Klaus-Dieter Budras, Romay A. Carretero, Gerhard Forstenpointner, Corinna Klupiec, Johann Maierl, Maren Meiners, Ivan Míšek, Christoph K. W. Mülling, Beltrán M. Navarro, Alexander Probst, Sven Reese, Jesus Ruberte, Ingrid Walter, Gerald Weissengruber, and Grammatia Zengerling. Avian Anatomy: Textbook and Colour Atlas. Sheffield: 5M Publishing, 2016. Print.
The Embryonic lives of Spotted Salamanders
Over the past couple of weeks, the last of the spotted salamander larvae around Ithaca have left their eggs and are now swimming around in vernal pools, pools that form in the spring and dry up later in the year. They will feed and grow in these pools until they become adult salamanders and adopt a terrestrial lifestyle. To the larvae, their lives have just begun, but to an outside viewer, a lot has already happened.
Back in April, Jonah Marion (’20) wrote a blog post about the spotted salamander migration in Ithaca, which occurred on May 29. In the rain and under the cover of darkness, the salamanders had migrated from their forest habitat to the vernal pools where they reproduce. The salamander migration is just the beginning of a fascinating life history. With camera in hand, I have been continually checking up on the spotted salamanders and their embryonic offspring throughout the season.
Breeding
The spotted salamander breeds in vernal pools which are free of predatory fish since they dry up later in the year. Because adult salamanders are normally terrestrial, they have lungs, not gills. Thus, during breeding, they have to return to the surface of the water every few minutes to breathe. Despite being terrestrial most of the year, the salamanders are well adapted to swimming; with the help of their muscular tails, they can propel themselves through the water by moving in an S-shaped pattern.
During breeding itself, the males deposit sperm-filled spermatophores, which females pick up and store in their spermathecae. The spermathecae are organs used to store sperm for later use, sometimes even for future breeding seasons.1 When the females are ready, they will use their stored sperm to fertilize their eggs, and then deposit them onto sturdy pieces of vegetation.
After breeding, the adult salamanders return to land to continue their terrestrial lifestyle. The migration from their vernal pools doesn’t occur as simultaneously as the migration into their vernal pools; males can leave earlier than females since they don’t have to lay eggs, and individuals don’t all necessarily leave at the same time.
The Eggs

A spotted salamander egg mass secured to a branch in a vernal pool. The eggs are collectively surrounded by a thick protective jelly.
Spotted Salamander egg masses are wrapped around sturdy objects such the living branches of woody plants. The individual eggs are all packed within an outer gelatinous coat that protects them from predation. However, this thick coat makes it difficult for oxygen to diffuse to the developing embryos. To solve this problem, spotted salamanders have developed a symbiotic relationship with a type of green algae, Oophila amblystomatis. The algae grows within the individual eggs, producing oxygen through photosynthesis while acquiring nutrients from the embryonic waste products.2,3 More recent research has shown that these algae invade the embryonic salamander cells themselves and then disappear during later stages of development.4 This represents a unique case of endosymbiosis between a vertebrate and an alga which is still the subject of active research.5

~Five week old embryos. The eggs can be seen filled with symbiotic green algae.

A diving beetle larva standing on top of a mature egg mass. The thick outer jelly protects the salamander embryos from this predator.
The Larvae

Two larvae, days before hatching. You can make out their eyes and the black spots of pigment covering their skin. Some of their nest-mates have already left.
The pace of embryonic development can vary between populations and between egg masses; usually it takes 4 to 7 weeks for the larvae to finally leave their eggs. For the Ithaca population it took about 7 weeks, with some variation between and even within egg masses.
When I checked on the egg masses in late May some of them were completely empty, with the eggs inside broken open. This was a sure sign that the larvae had outgrown their eggs and had taken refuge among the abundant leaf litter. Larval salamanders are adapted to life in their vernal pools; they have external gills and no legs. They will feed and grow in these pools for 2 to 4 months until they metamorphosize into adult terrestrial salamanders. Then, they will move onto land to seek permanent shelter in the forest. It may take 2 to 3 years before they become sexually mature, and the cycle can start all over again.
References
- Chandler, C. H., and K. R. Zamudio. “Reproductive success by large, closely related males facilitated by sperm storage in an aggregate breeding amphibian.” Molecular Ecology 17.6 (2008): 1564-1576.
- Bachmann, Marilyn D., et al. “Symbiosis between salamander eggs and green algae: microelectrode measurements inside eggs demonstrate effect of photosynthesis on oxygen concentration.” Canadian Journal of Zoology 64.7 (1986): 1586-1588.
- Pinder, A., and S. Friet. “Oxygen transport in egg masses of the amphibians Rana sylvatica and Ambystoma maculatum: convection, diffusion and oxygen production by algae.” Journal of Experimental Biology 197.1 (1994): 17-30.
- Kerney, Ryan, et al. “Intracellular invasion of green algae in a salamander host.” Proceedings of the National Academy of Sciences 108.16 (2011): 6497-6502.
- Kerney, Ryan, John Burns, and Eunsoo KIM. “Investigating Mechanisms of Algal Entry into Salamander Cells.” Algal and cyanobacteria symbioses. 2017. 209-239.
Lunch Lecture: “What can we really do about emerging amphibian diseases”
 Wildlife Disease Association and Pathology Club are proud to present Wildlife pathologist, Dr. María Forzán, who will speak about emerging amphibian diseases. This lecture will focus on salamander chytrid (Batrachochytrium salamandrivorans) as a potential threat to North American Species.
Wildlife Disease Association and Pathology Club are proud to present Wildlife pathologist, Dr. María Forzán, who will speak about emerging amphibian diseases. This lecture will focus on salamander chytrid (Batrachochytrium salamandrivorans) as a potential threat to North American Species.
Thai food will be served!
Please remember to bring your own plates and utensils!