by Joe Gregoire, Warwick Senior Master Gardener Volunteer
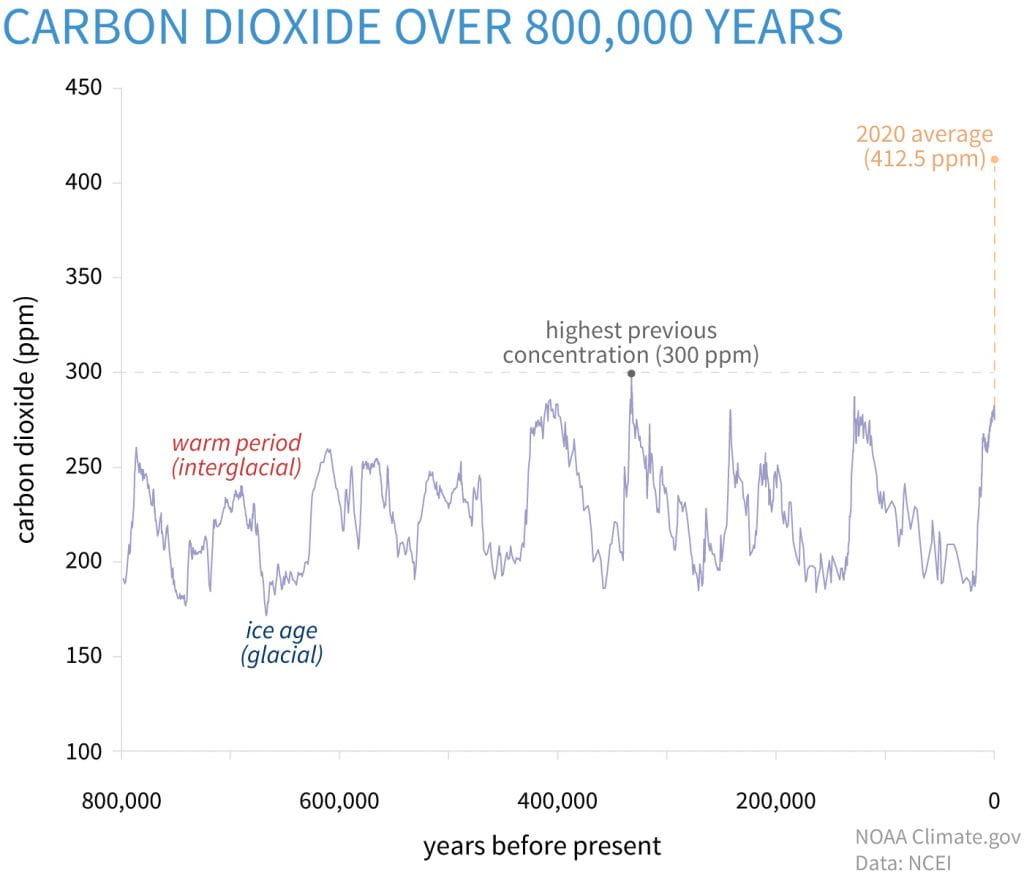 For decades, we’ve been involved in a global debate about the warming of the planet and the resulting climate change that impacts us all. From heat waves that fuel drought and wildfires to extreme cold that shortens growing seasons and puts food production at risk across the planet, the increasing unpredictability of climate has an impact on the lives of billions of people each year, making the debate over the causes of climate change less and less relevant. It is like debating over the cause of a house fire, while the house is burning all around us. While opinions continue to be polarized on the topic of human or natural causes of climate change, the science behind what is increasing the temperature of the planet is clear. The increase in parts per million of carbon dioxide is linked to the continuing trend in rising global temperatures.
For decades, we’ve been involved in a global debate about the warming of the planet and the resulting climate change that impacts us all. From heat waves that fuel drought and wildfires to extreme cold that shortens growing seasons and puts food production at risk across the planet, the increasing unpredictability of climate has an impact on the lives of billions of people each year, making the debate over the causes of climate change less and less relevant. It is like debating over the cause of a house fire, while the house is burning all around us. While opinions continue to be polarized on the topic of human or natural causes of climate change, the science behind what is increasing the temperature of the planet is clear. The increase in parts per million of carbon dioxide is linked to the continuing trend in rising global temperatures.
 As an avid gardener, managing the variability of the weather is a constant part of the experience when growing food and I regularly reflect on the global phenomenon and what I can do to protect my crops from the risks that nature can bring. Floating row covers protect my plants from frost damage in spring and fall. A three inch layer of compost mulch over my garden beds retains moisture in the soil and captures rainfall like a sponge, helping my plants persist through dry spells. And shading my garden soil with a canopy of growing plants, provides my plants with a microclimate of cooler soil temperatures during the hottest days of summer. I’ve come to appreciate that there are actions I can take in my own garden that can increase my plant health and the resulting yield from my crops at harvest time. I’ve come to believe that we can take action to develop solutions that work with nature as the best path to follow in my gardening pursuits.
As an avid gardener, managing the variability of the weather is a constant part of the experience when growing food and I regularly reflect on the global phenomenon and what I can do to protect my crops from the risks that nature can bring. Floating row covers protect my plants from frost damage in spring and fall. A three inch layer of compost mulch over my garden beds retains moisture in the soil and captures rainfall like a sponge, helping my plants persist through dry spells. And shading my garden soil with a canopy of growing plants, provides my plants with a microclimate of cooler soil temperatures during the hottest days of summer. I’ve come to appreciate that there are actions I can take in my own garden that can increase my plant health and the resulting yield from my crops at harvest time. I’ve come to believe that we can take action to develop solutions that work with nature as the best path to follow in my gardening pursuits.
 In her book, The Soil Will Save Us – How Scientists, Farmers, and Foodies are Healing the Soil to Save the Planet, author Kristin Ohlson delivers a powerful message on the subject of climate change. Looking past the debate over whether humanity is the cause of global warming or not, Ohlson dives deep into the science of soil health and its connection to the carbon cycle at a macro level. Through her travels around the world researching the topic, Ohlson finds numerous best practices in soil science, farming and ranching practices, and the food community that drives the demand for sustainable agriculture. She successfully builds the case for humanity as a solution to climate change, working with nature to reduce carbon from the atmosphere.
In her book, The Soil Will Save Us – How Scientists, Farmers, and Foodies are Healing the Soil to Save the Planet, author Kristin Ohlson delivers a powerful message on the subject of climate change. Looking past the debate over whether humanity is the cause of global warming or not, Ohlson dives deep into the science of soil health and its connection to the carbon cycle at a macro level. Through her travels around the world researching the topic, Ohlson finds numerous best practices in soil science, farming and ranching practices, and the food community that drives the demand for sustainable agriculture. She successfully builds the case for humanity as a solution to climate change, working with nature to reduce carbon from the atmosphere.
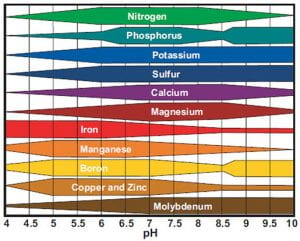
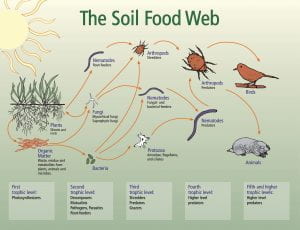 At the root of the solution to global warming, is the soil beneath our feet. And the healthier the soil, the better. Healthy soil is more than just its composition and the nutrients it contains. Healthy soil is healthy because of the life within in it, in the form of microscopic bacteria, fungi, and insects which comprise the soil food web. A virtuous cycle of carbon capture from the atmosphere into the soil, the soil food web and our understanding of it is emerging as a keystone solution to reducing CO2 levels in the atmosphere.
At the root of the solution to global warming, is the soil beneath our feet. And the healthier the soil, the better. Healthy soil is more than just its composition and the nutrients it contains. Healthy soil is healthy because of the life within in it, in the form of microscopic bacteria, fungi, and insects which comprise the soil food web. A virtuous cycle of carbon capture from the atmosphere into the soil, the soil food web and our understanding of it is emerging as a keystone solution to reducing CO2 levels in the atmosphere.
New research sheds light on the fascinating communication process that exists between growing plants and the microbial life in the soil with plants “signaling” their need for various nutrients by attracting microbial life to their root zone using exuded sugars created through photosynthesis. While we’ve known that photosynthesis enables plants to produce their own food by transforming sunlight and atmospheric CO2 into carbon sugars that feed growth and release oxygen back into the atmosphere, new research is showing that these carbon sugars are used by plants to grow the soil life surrounding their roots at the same time, by exuding sugars into the soil through their roots. These sugars in the soil attract beneficial soil bacteria and fungi that consume the sugars and grow in number as a result. Nematodes and other microbial life then feed on the growing bacteria and fungi populations and release plant soluble nutrients through their waste into the plant root zone, like microscopic herds of cattle dropping their manure for the benefit of the plants in a pasture. And the extensive microscopic network of mycelium that are the living fungi in the soil, trade those same root exudate sugars with moisture and minerals they extract from far beyond the reach of plant roots. All of this exudate sugar, carbon that was once atmospheric CO2, remains in soil that is undisturbed and kept covered by mulch or growing plants and protected from the oxidation that would combine it with oxygen and release it back into the atmosphere as CO2.
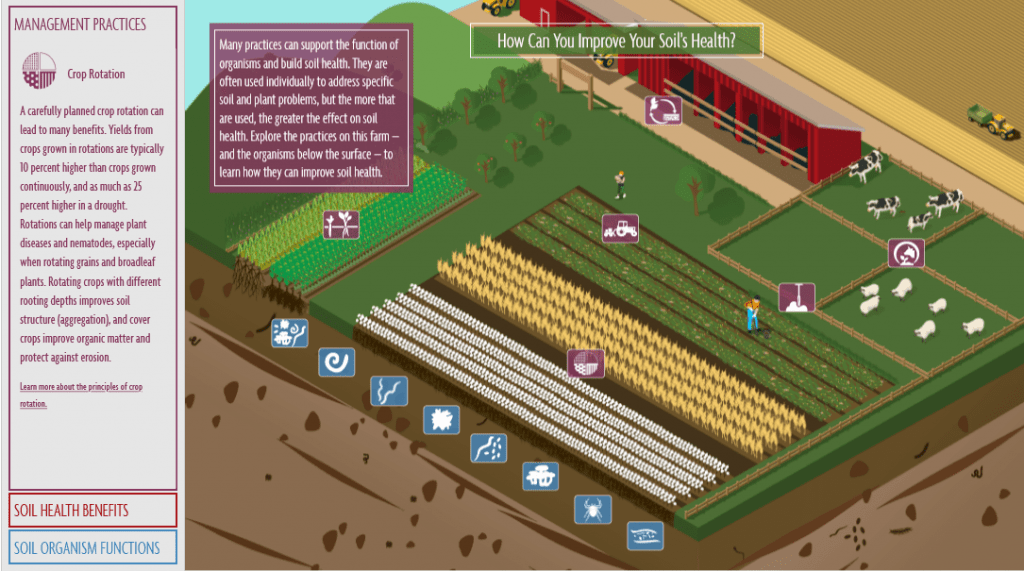 With this growing understanding of soil life and the benefit of carbon capture that is inherent in the natural process of the soil food web, Ohlson uncovers a growing movement of collaboration between environmentalists and agriculturalists that, not long ago, was an adversarial relationship. Traditional environmentalist attitudes called for humanity to leave nature alone is evolving into a movement that sees the potential for humanity to work with nature in a beneficial way. And traditional agriculturalist attitudes that look at nature as something that needs to be tamed and controlled through tilling, chemical fertility and pest elimination and monocropping for production efficiency, is evolving into a movement that sees natural processes as a solution to sustainable food production and increased yields. And as these two once opposing movements evolve, they are coming together in collaborative efforts to protect nature through the use of agriculture that can have rapid benefits to thousands and thousands of acres of cropland around the world.
With this growing understanding of soil life and the benefit of carbon capture that is inherent in the natural process of the soil food web, Ohlson uncovers a growing movement of collaboration between environmentalists and agriculturalists that, not long ago, was an adversarial relationship. Traditional environmentalist attitudes called for humanity to leave nature alone is evolving into a movement that sees the potential for humanity to work with nature in a beneficial way. And traditional agriculturalist attitudes that look at nature as something that needs to be tamed and controlled through tilling, chemical fertility and pest elimination and monocropping for production efficiency, is evolving into a movement that sees natural processes as a solution to sustainable food production and increased yields. And as these two once opposing movements evolve, they are coming together in collaborative efforts to protect nature through the use of agriculture that can have rapid benefits to thousands and thousands of acres of cropland around the world.

Intensive grazing of livestock using electric fencing to corral livestock into a tight herd and practicing rotational grazing through managing the movement of these herds over a pastureland, replicates the natural behavior of herding livestock that evolved to graze in tight herds as protection from predators that no longer pose a natural threat. This intensive grazing behavior is what created the deep soils that covered the Great Plains prior to European settlement and that were blown away with the ravages of the dust bowl. And a movement away from vast acres of monocrop corn and soil bean production toward no-till multispecies crop production and cover cropping, accelerates the introduction of carbon sugars into the soil food web. For an increasing number of practicing growers, this is increasing their yields, lowering their costs, and restoring the moisture retention, erosion protection, and soil health of their land. All the while, acting as a viable solution to reducing CO2 levels within the atmosphere.
If you find this subject interesting and would like to learn more about how our daily food choices can play an active role in collaborating with nature as a solution to the global warming problem, then I highly recommend taking time during these last days of winter to read this book.