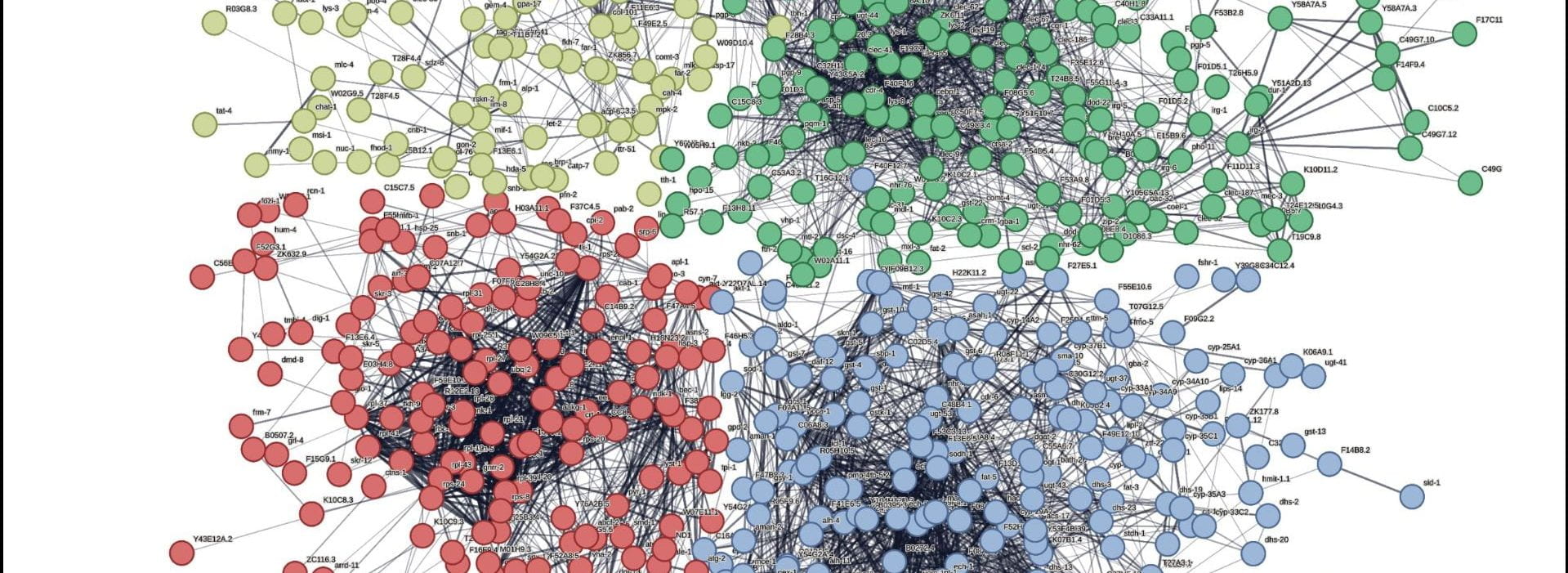
Elaborate signaling mechanisms coordinate reproduction and metabolism, which can have profound effects on longevity.
C. elegans, the model system we study in the lab, are hermaphrodites and self-fertile. Previous studies have implicated communications between the germline and the intestine, a major metabolic tissue in worms, to regulate lipid metabolism and affect longevity. To further investigate this interesting inter-tissue signaling mechanism, we took advantage of several types of self-sterile mutants: mutants lacking germ cells (germlineless), mutants lacking sperms (feminized), mutants lacking oocytes (masculinized). We employ genetic, genomic, and metabolomic strategies, to compare and contrast how different ways of disrupting the reproductive system impact fat metabolism and longevity. We are particularly excited to take advantage of the masculinized mutant to study how a male-derived signaling pathway modulates longevity. We are also excited to exploit the feminized mutant to study how the nuclear hormone receptor NHR-49 regulates oogenesis and oocyte activation.