This would also be the optimum timing for placing CM mating disruption (MD) dispensers in orchard blocks of 5-acres or more. MD will reduce mate finding leading to reduced viable egg laying and worm injury. The use of MD in problem orchards will reduce the pressure placed solely on the use of insecticide programs to control the pest. A number of products are available for use in New York State. In blocks of high fruit infestation from codling moth in 2017, a possible cause may have been the use of materials ineffective against the worm during the three generation emergence periods of the larval stage, most notably the 3rd generation in September. The use of Actara (thiamethoxam) for plum curculio at PF, 1st or 2nd cover will not have provided commercial control of the 1st generation CM. The possibility of frequently used insecticides slipping is also a possibility.
Introduction: It can be argued that of the internal worm complex, the codling moth has historically been the greatest threat to tree fruit. This insect continues to re-emerge as a primary pest of apple throughout the world. During the 2016 harvest we observed very high levels of fruit infestation in Hudson Valley apple, ranging from 2% to 40% injury in commercial production.
The insect is now overwintering as full-grown larvae, residing within a silken cocoons, tucked under inaccessible bark scales, in soil or hidden in the leaves and debris around the base of trees. The larvae will pupate and emerge from their cocoon early in the spring as an adult moths. The range of first flight begins from ate April to early May this season due to a cool spring, with first hatch beginning late May to early June.
Adult flight is now beginning. The use of pheromone traps are the growers best indication of first flight on their farms while providing adult male numbers to understand population density in specific blocks. Temperatures below 60°F, which we have been experiencing in the Hudson Valley, impede male activity and prevent mating, so a cooler spring will delay significant egg hatch for the first generation. NEWA weather data is available to help predict this, incorporated into the degree day model. The moth has been observed to produce two peaks during the 1st generation, often extending its flight well into the summer. We base our management of the insect on codling moth larval emergence using a predictive degree day model of 220 degree days from CM adult first flight. Two applications, which often start at 1st cover, are needed to cover the emergence period in blocks where CM has caused historical fruit injury. The 2nd application often (but not always) made on the heels of 1st generation obliquebanded leafroller emergence.
Mating disruption (MD) of codling moth is currently used in 90 percent of all apples and pears grown in Washington State, with relatively few acres (but on the increase) using MD for codling moth in NYS. Details on the implimentation of MD can be found in the article titled “Managing Codling Moth and Oriental Fruit Moth In Apples“, by Deborah Breth, CCE, LOF IPM Specialist & Team Leader; Art Agnello, Dept. of Entomology, Cornell University; Elizabeth Tee, CCE -LOF Program Aide. Mating disruption options listed can be obtained from pest management distributors that include nIsomate-CM/OFM Twin Ties (TT) employed at 200 ties/ acre, Checkmate CM-F 14.3S used at 2.4-4.8 fl.oz./acre and Checkmate CM-OFM Duel using 150-200 dispensers/ acre.
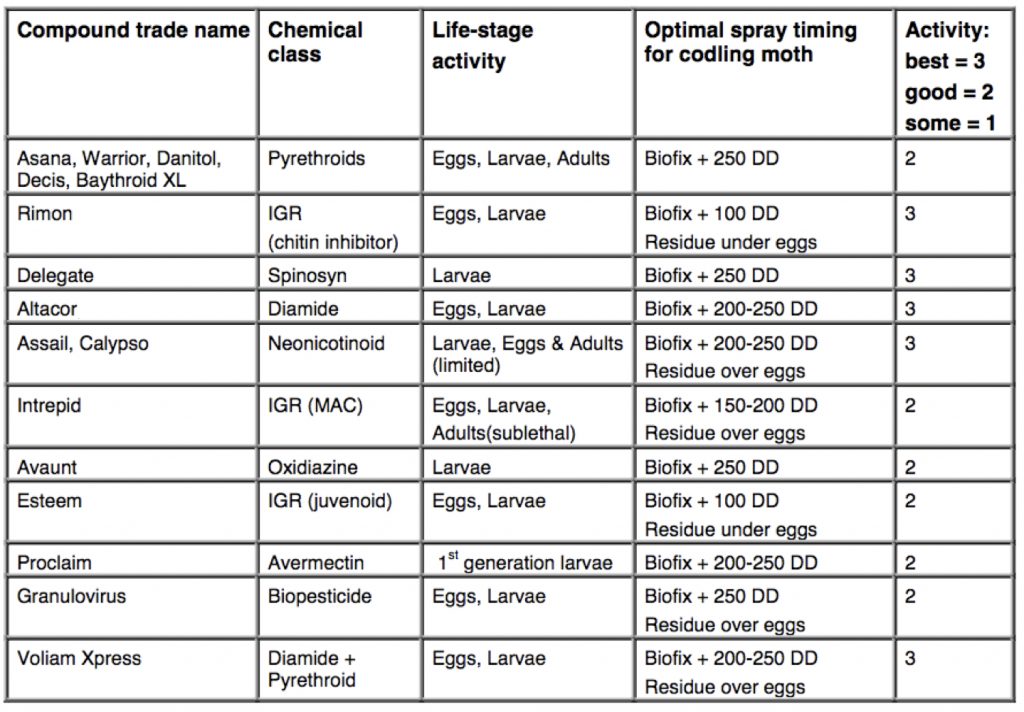
secticides used for CM control.
(Wise, MSU)
An additional option is to apply a granulosis virus formulation at 200-250 DD 50°F. High moth pressure requires 2-3 sprays for the first generation, but in lower pressure orchards (with counts of less than 5 moths per trap per week), you can control CM with a single spray timed at 350 DD 50°F.Codling moth granulosis virus. Formulations include Cyd-X, Cyd-X HP, Madex HP by Certis; Carpovirusine by Arysta LifeScience. Cyd-X 0.06SC is applied @ 0.25-0.4 qt/acre and Carpovirusine 0.99SC @ 0.25-0.4 qt/acre.
Codling Moth Granulosis Virus contains an insecticidal baculovirus, Cydia pomonella granulovirus, which is specific to the larval form of the codling moth, and is registered for use in apples, pears, and (Cyd-X only) plums. This biological insecticide must be ingested in order to be effective, after which the viral occlusion bodies dissolve in the larval midgut and release infectious virions. These enter the cells lining the digestive tract, where they replicate; eventually, the other tissues are infected and the larva stops feeding and eventually (within 3-7 days) dies. After death, the larva disintegrates, releasing billions of new occlusion bodies, which may infect other codling moth larvae upon ingestion. No adverse effect to fish, wildlife or beneficial organisms has been observed; it has a low bee-poisoning hazard.
Insecticide Resistance Development in Codling Moth:
The organophosphate class of insecticides, including Guthion (azinphos-methyl) and Imidan (phosmet) have been used since the 1960’s, for over 50 years, to manage the codling moth. The development of resistance by codling moth to Imidan is likely if its reoccurring use has been for Plum Curculio and or OBLR management during mid-summer. Consistent use during the past ten years or more increasing resistance potential. Those specific timings would provide some level of control of CM while providing various rates of residual exposure. Low rates of residual increase the selection for resistance during the early and mid-summer generations of the pest. A scenario, such as the switch from Guthion to Imidan in season long program use would affect the same target site (nerve receptor sites for ACh, acetylcholinesterase) within the insect to contribute to resistance.
In the Hudson Valley to date I don’t believe resistant strains are widespread, even though we are hearing of increasing reports of CM damage to tree fruit. The broad availability and use of insecticides that include the different IRAC classes of active ingredients for plum curculio from PF to 2nd cover and mid-summer management of OBLR, would reduce region-wide resistance of CM to any one specific insecticide class. We have seen the use of these materials to include Avaunt, Exirel, Calypso, Carbaryl, pre-mixes including pyrethroids or pyrethroids alone. Insecticides of various classes for the overwintering OBLR used at PF would likely impact CM to a lesser degree when used at petal fall timing.
There haven’t been recent studies to detect phosmet resistance to CM in NY that I’m aware of. In Michigan, a study in 2008 detected a 7-8 fold level of resistance in orchard site specific CM populations to codling moth (see abstract from work done by John Wise in Mich. State below). However, no resistance to acetamiprid (Assail) and spinosad (Delegate, Spintor, Entrust) was detected in the study.
That said, it’s very likely we have codling moth populations resistant to older insecticide classes, including pyrethroids and OP’s in orchards throughout the Northeast.
Insecticide rotation: I would suggest the use of specific materials for CM be employed during 1st-2nd cover and again in mid-July, when model predictions for larva emergence are called for AND in orchards with reoccurring fruit injury from CM. The use of Assail to manage apple maggot during 2nd generation CM larval emergence has been a good option, as it’s been shown to be one of the better materials against both CM & AM. The use of Delegate, Altacor at 1st – 2nd cover would reduce the resistance potential while picking up a few lingering overwintering OBLR. Mating disruption and granulosis virus are also good choices in conventional and organic production systems.
Remember to rotate classes for EACH GENERATION and not each spray during a generation, to reduce the potential for insecticide resistance.
Mota-Sanchez D1, Wise JC, Poppen RV, Gut LJ, Hollingworth RM.
The codling moth is one of the principal pests of apple in the world. Resistance monitoring is crucial to the effective management of resistance in codling moth. Three populations of codling moth in neonate larvae were evaluated for resistance to seven insecticides via diet bioassays, and compared with a susceptible population. In addition, apple plots were treated with labeled field rate doses of four insecticides. Treated fruit were exposed to neonate larvae of two populations from commercial orchards.
RESULTS: Two populations of codling moth expressed two- and five fold resistance to azinphos-methyl, seven- and eight fold resistance to phosmet, six- and tenfold resistance to lambda-cyhalothrin, 14- and 16-fold resistance to methoxyfenozide and sixfold resistance to indoxacarb, but no resistance to acetamiprid and spinosad. The impact of the resistance to azinphos-methyl, measured as fruit damage, increased as the insecticide residues aged in the field. In contrast, fruit damage in methoxyfenozide- and lambda-cyhalothrin-treated fruit was observed earlier for resistant codling moth. No differences in efficacy were found for acetamiprid.CONCLUSIONS: Broad-spectrum insecticide resistance was detected for codling moth. Resistance to azinphos-methyl, lambda-cyhalothrin and methoxyfenozide was associated with reduced residual activity in the field. Broad-spectrum resistance presents serious problems for management of the codling moth in Michigan.