Electrophoresis, 2012: Microfluidic transport in Microdevices for Rare Cell Capture
Abstract: The isolation and capture of rare cells is a problem uniquely suited to microfluidic devices, in which geometries on the cellular length scale can be engineered and a wide range of chemical functionalizations can be implemented. The performance of such devices is primarily affected by the chemical interaction between the cell and the capture surface and the mechanics of cell-surface collision and adhesion. As rare cell-capture technology has been summarized elsewhere (E. D. Pratt et al., Chem. Eng. Sci. 2011, 66, 1508–1522), this article focuses on the fundamental adhesion and transport mechanisms in rare cell-capture microdevices, and explores modern device design strategies in a transport context. The biorheology and engineering parameters of cell adhesion are defined; adhesion models and reaction kinetics briefly reviewed. Transport at the microscale, including diffusion and steric interactions that result in cell motion across streamlines, is discussed. The review concludes by discussing design strategies with a focus on leveraging the underlying transport phenomena to maximize device performance.
Figures:
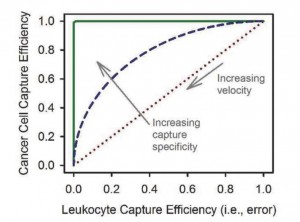
- An example of a receiver operating characteristic (ROC) curve, which shows the sensitivity of a rare cell-capture device; in this example we consider only cancer cell capture versus capture of leukocytes, the most common contaminant. A given geometry, antibody, and velocity result in a balance between cancer cell capture efficiency (true positives) and leukocyte capture efficiency (false positives). A practical device (blue dashes) has an ROC curve between pure chance (red dots) and a theoretical perfect test (green line).
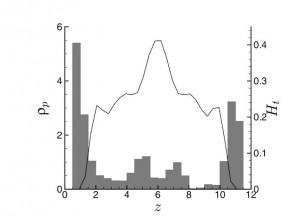
- Margination causes increased platelet density (ρp, shaded bars) at the walls (z = 0 and 12, respectively), while erythrocytes are concentrated in the center of the channel, as measured by the blood hematocrit (Ht, solid line). Adapted from [1].
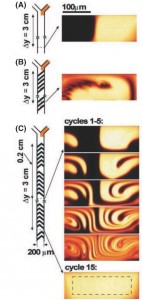
- Cross sections of the dye distribution in a microfluidic channel designed to create staggered, time-dependent whorls or twist maps. From [2]; used with permission.
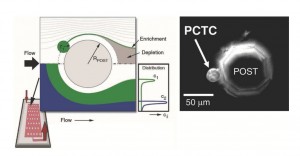
- Distribution of two populations of particles approaching a circular obstacle. Local streamline distortion enhances the collision of particles with the obstacle, increasing efficiency in cell-capture systems (left). A prostate circulating tumor cell (PCTC) captured on an octagonal obstacle post (right) [3].
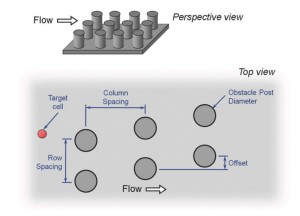
- An obstacle array’s rational geometry lends itself to parametric engineering optimization.
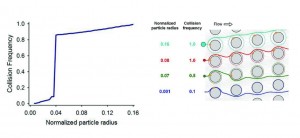
- Collision frequency versus particle size in a GEDI obstacle array (left). The sharp transition between high and low collision frequencies is also made evident by comparing particle trajectories (right). Size-dependent collision dynamics, combined with a specific immunocoated surface, maximize both efficiency and purity.
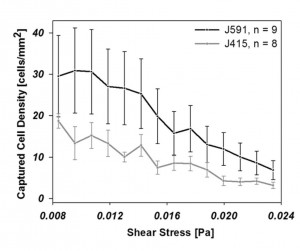
- LNCaP cell capture rates as a function of shear stress and capture surface immunochemistry. J415 and J591 have similar affinities [4] but differences in binding site location result in J591 (located at the protein’s apical domain) outperforming J415 (located near the transmembrane domain). Adapted from [5], with permission.
Citation: Smith JP, Barbati AC,Santana SM, Gleghorn JP, Kirby BJ. Microfluidic Transport in Microdevices for Rare Cell Capture, Electrophoresis, vol(33): pages 3133-3142. doi