J.M.Iacchei
Each year I am given the opportunity to pursue professional development relevant to my position in Conservation. This support for continued growth is an invaluable benefit. This year, I decided to start working on one of my most challenging obstacles in Conservation (and Academia): Chemistry.
It is easy to get lost in the daily work – surface cleaning, stabilization, humidification, etc. that are familiar and routine – and lose sight of the underlying chemical principles that are ever present in our treatment decision making. I felt that getting a grasp of these principles was essential to my professional growth. I wanted to better understand why I was carrying out treatments and be able to apply that understanding to decision-making of items and materials that were not familiar and treatments that were not routine.
With Chemistry being well beyond my comfort zone, diving into a college level Chemistry, 18 years after my last Chemistry course, was a little too ambitious. Instead, I chose to apply to the Chemistry for Conservators correspondence course offered through International Academic Projects. It is designed for those of us who do not have a strong background in Chemistry, but who work closely with it every day.
The course distilled Chemistry down to the fundamental principles that directly impact conservation practices – and it took out “the math”. It is divided into four blocks to be completed over 4 months, each block building upon the last as new topics are introduced. Block 1 introduced the physical world with focus on air and water; Block 2 covered basic chemical principles – atoms, electrons, compounds, reactions, molecular models; Block 3 began to link the principles introduced in Blocks 1 and 2 to deeper concepts – solutions, electrochemical principles, organic compounds, polymers; and Block 4 addressed the challenges conservators are presented with most often – the effects of water, cleaning –why, when, and how much; adhesives, and degradation. Each of these 4 Blocks was accompanied by readings from the textbook (Chemistry 2nd ed.), the Science for Conservators Series, Volumes 1-3, and supplemental course notes accompanying each block, as well as experiments (materials supplied) and review questions. The textbook provided a general introduction to the topics covered, the Science for Conservator Series and course notes provided a more technical explanation, the questions highlighted key concept, and the experiments provided a concrete visual example of the concepts discussed in the texts.
Though simple, I found the experiments required the most time and independent thought. They provided a means to practice those skills needed in conservation assessment and decision making: observation, organization of thought, ability to draw conclusions, and direct application of understanding gained from drawn conclusions.
This course was challenging, but manageable. It is noted that is a time intensive course and to plan for 10-12 hours/week to devote to the material. This is fairly accurate – less if you are a quick reader, and Chemistry comes naturally to you; more if you are a slow reader, like to take meticulous notes, need to re-read, and Chemistry is not you forte. The course covered topics across conservation – extending beyond the conditions found with paper and photographic collections with which I am most familiar. Metal, ceramic, glass, and textile materials, have been surfacing more and more often in the conservation lab from Cornell University Library Collections. Having some framework and resources will be helpful in understanding current conditions and guiding treatment needs. With this course, I am better equipped to take a more informed approach to the treatment of the materials I am responsible for preserving. And while there is more to learn, I left this course with a foundation to build upon. I gained greater awareness of the underlying chemical principles that explain current conditions and the potential options and outcomes of material choices and treatment methods.
Here are two examples:
 Shown here is a tintype (silver image on black lacquered iron support) from the Loewentheil Family Photographic Collection in the Library’s Division of Rare and Manuscript Collections. It demonstrates how corrosion can occur if the tintype is exposed to poor environmental conditions. Rust is the slow oxidation of iron. It occurs as 2-part reaction when the iron support is exposed to BOTH air and water. First the iron is oxidized by the air to form iron oxide. The iron oxide then reacts with moisture in the air to produce hydrated iron oxide-more commonly known as rust. By minimizing one of the two factors causing rust to occur- exposure to air or water/moisture in the air- you can assist their preservation.
Shown here is a tintype (silver image on black lacquered iron support) from the Loewentheil Family Photographic Collection in the Library’s Division of Rare and Manuscript Collections. It demonstrates how corrosion can occur if the tintype is exposed to poor environmental conditions. Rust is the slow oxidation of iron. It occurs as 2-part reaction when the iron support is exposed to BOTH air and water. First the iron is oxidized by the air to form iron oxide. The iron oxide then reacts with moisture in the air to produce hydrated iron oxide-more commonly known as rust. By minimizing one of the two factors causing rust to occur- exposure to air or water/moisture in the air- you can assist their preservation.
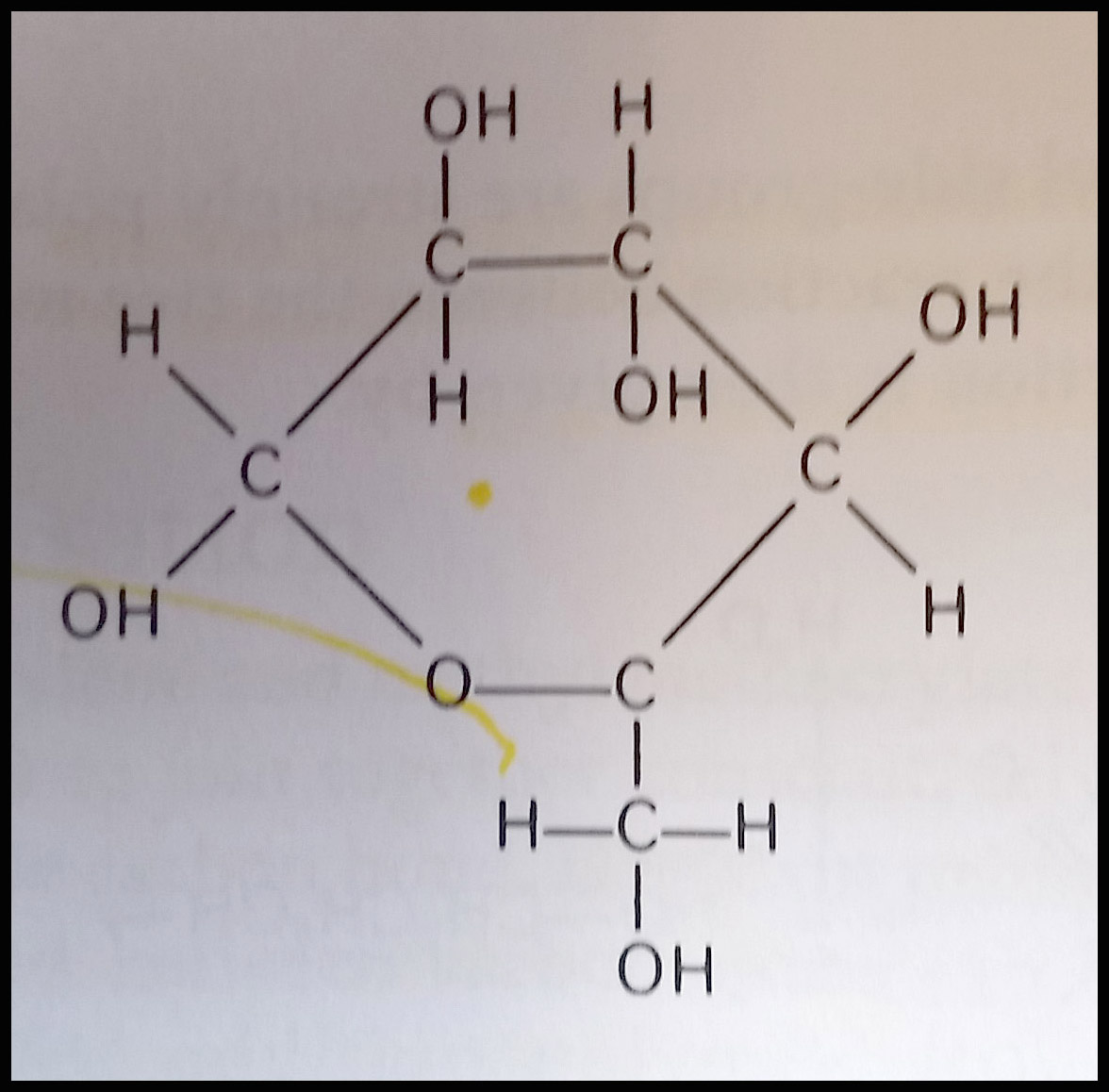
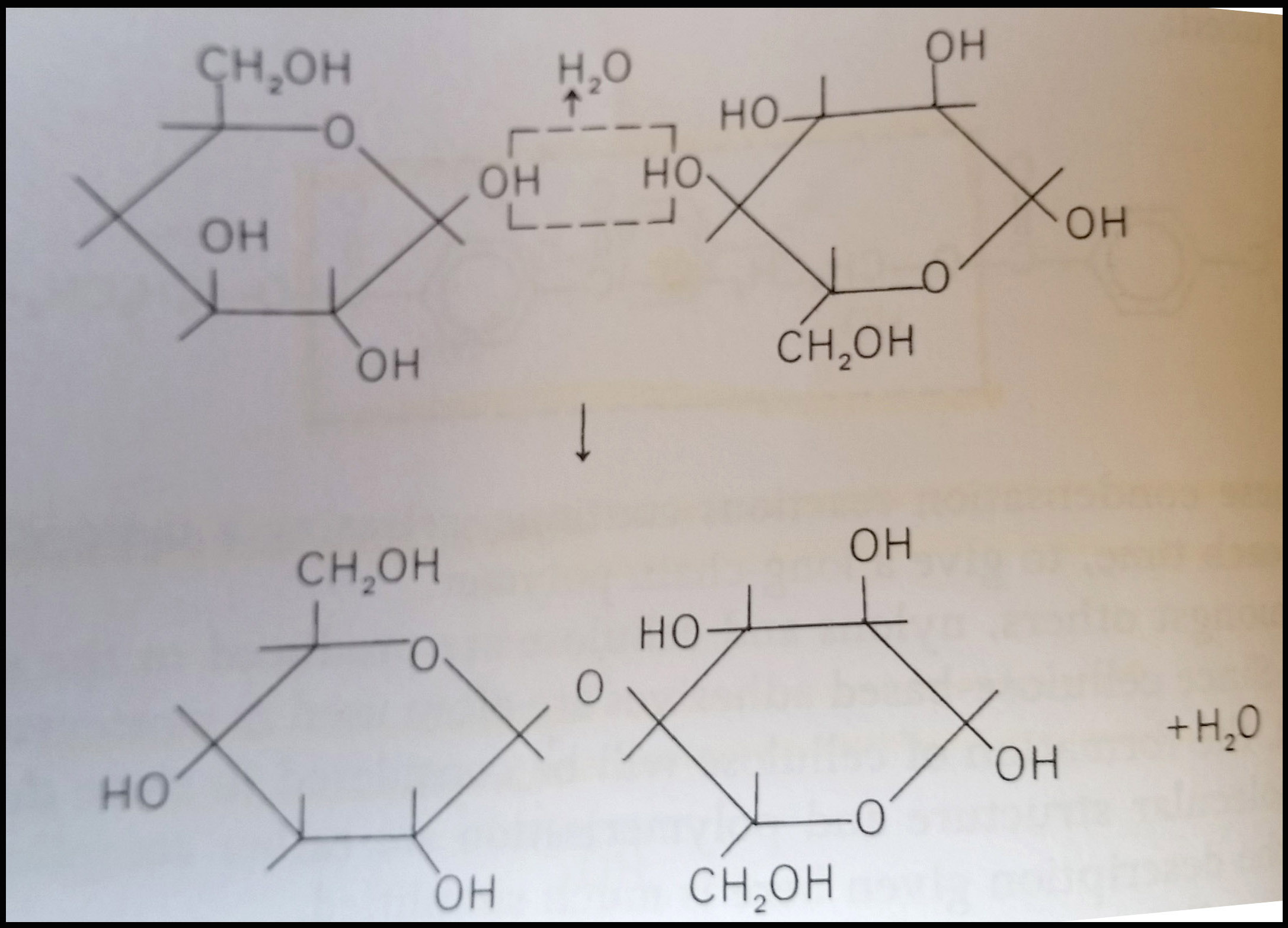
 Late 19th and early 20th century newspapers provide excellent examples of cellulose deterioration caused by acid hydrolysis. Cellulose in a polymer of glucose which forms from condensation reactions that occur between the reactive -OH (hydroxyl) side groups.
Late 19th and early 20th century newspapers provide excellent examples of cellulose deterioration caused by acid hydrolysis. Cellulose in a polymer of glucose which forms from condensation reactions that occur between the reactive -OH (hydroxyl) side groups.
One of the ways these cellulose chains are broken down is by acid hydrolysis. In the presence of moisture, acids from the environment (air pollution or poor quality enclosures) or from within the paper (raw materials, manufacturing processes) repeatedly cut the glucose chains into shorter lengths. This reaction also produces more acids – providing fuel for further reactions and continued degradation. This newspaper shows the affects of this deterioration–brittleness, loss of strength, crumbly edges, and darkening. This course provided me with an excellent background to navigate the variety of approaches to mitigating these concerns–treatment, environment, and enclosures.
I am grateful to Cornell University Library, Tre Berney, Director Digitization and Conservation Services and Michele Hamill, Paper and Photograph Conservator, for their continued support and encouragement of my professional development, and the rather generous amount of time I was given to spend on this coursework.