Biomicrofluidics, 2014: Characterization of microfluidic shear-dependent epithelial cell adhesion molecule immunocapture and enrichment of pancreatic cancer cells from blood cells with dielectrophoresis
Citation: Huang C, Smith JP, Saha TN, Rhim AD, Kirby BJ. Characterization of microfluidic shear-dependent
epithelial cell adhesion molecule immunocapture
and enrichment of pancreatic cancer cells
from blood cells with dielectrophoresis, Biomicrofluidics, 8, 044107 2014. doi pdf
Abstract: Current microfluidic techniques for isolating circulating tumor cells (CTCs) from cancer patient blood are limited by low capture purity, and dielectrophoresis (DEP) has the potential to complement existing immunocapture techniques to improve capture performance. We present a hybrid DEP and immunocapture Hele-Shaw flow cell to characterize DEP’s effects on immunocapture of pancreatic cancer cells (Capan-1, PANC-1, and BxPC-3) and peripheral blood mononuclear cells (PBMCs) with an anti-EpCAM (epithelial cell adhesion molecule) antibody. By carefully specifying the applied electric field frequency, we demonstrate that pancreatic cancer cells are attracted to immunocapture surfaces by positive DEP whereas PBMCs are repelled by negative DEP. Using an exponential capture model to interpret our capture data, we show that immunocapture performance is dependent on the applied DEP force sign and magnitude, cell surface EpCAM expression level, and shear stress experienced by cells flowing in the capture device. Our work suggests that DEP can not only repel contaminating blood cells but also enhance capture of cancer cell populations that are less likely to be captured by traditional immunocapture methods. This combination of DEP and immunocapture techniques to potentially increase CTC capture purity can facilitate subsequent biological analyses of captured CTCs and research on cancer metastasis and drug therapies.
Figures:
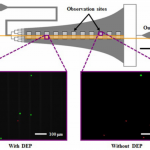
- FIG. 1. Schematic of the Hele-Shaw flow cell and its interdigitated electrodes with lead connections to an applied voltage (6V) and ground (GND), and elongated straight inlet channel compared to previous designs.35,37 The elongated straight inlet channel was 500 lm wide, the smaller branching channels were 156 lm wide, and all channels were 48 lm tall. The main chamber geometry leads to a monotonically decreasing shear stress along the device centerline, which allows for cell capture to be measured as a function of shear stress.40–42 Inset images show fluorescently labeled PANC-1 cells (green) and PBMCs (red) adhered to the antibody-functionalized surface with and without DEP effects. These example images show that at an applied AC electric field frequency of 200 kHz, more PANC-1 cells and fewer PBMCs were captured with DEP compared to without DEP. Captured cells in each pair of 1-mm2 observation windows were enumerated and compared at a series of observation sites corresponding to a range of shear stresses found in typical immunocapture devices.4,12,39,43
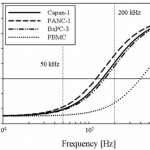
- FIG. 2. Predicted DEP response, as described by fCM, of Capan-1 (solid line), PANC-1 (dashed line), BxPC-3 (dasheddotted line), and PBMCs (dotted line) as a function of applied electric field frequency. Cells were modeled as single-shelled dielectric spheres, described by Eqs. (2) and (3). In a diluted PBS suspending medium with conductivity = 0.07 S/m, the crossover frequency was experimentally determined to be approximately 140 kHz for Capan-1, 120 kHz for PANC-1, 140 kHz for BxPC-3, and 400 kHz for PBMCs. Because the DEP force is weak in these frequency ranges and therefore difficult to observe, our crossover frequency estimates all have an uncertainty of 610 kHz. These empirical measurements, combined with Eq. (3), corresponded to specific membrane capacitance values of Cmembrane = 13.5 mF/m2 for Capan-1, Cmembrane = 14.5 mF/m2 for PANC-1, and Cmembrane = 15 mF/m2 for BxPC-3, and Cmembrane = 7.5 mF/m2 for PBMCs in the dielectric shell model. At 50 kHz, cancer cells and blood cells both exhibit a nDEP response; at 200 kHz, however, cancer cells exhibit a pDEP response whereas PBMCs still exhibit a nDEP response.
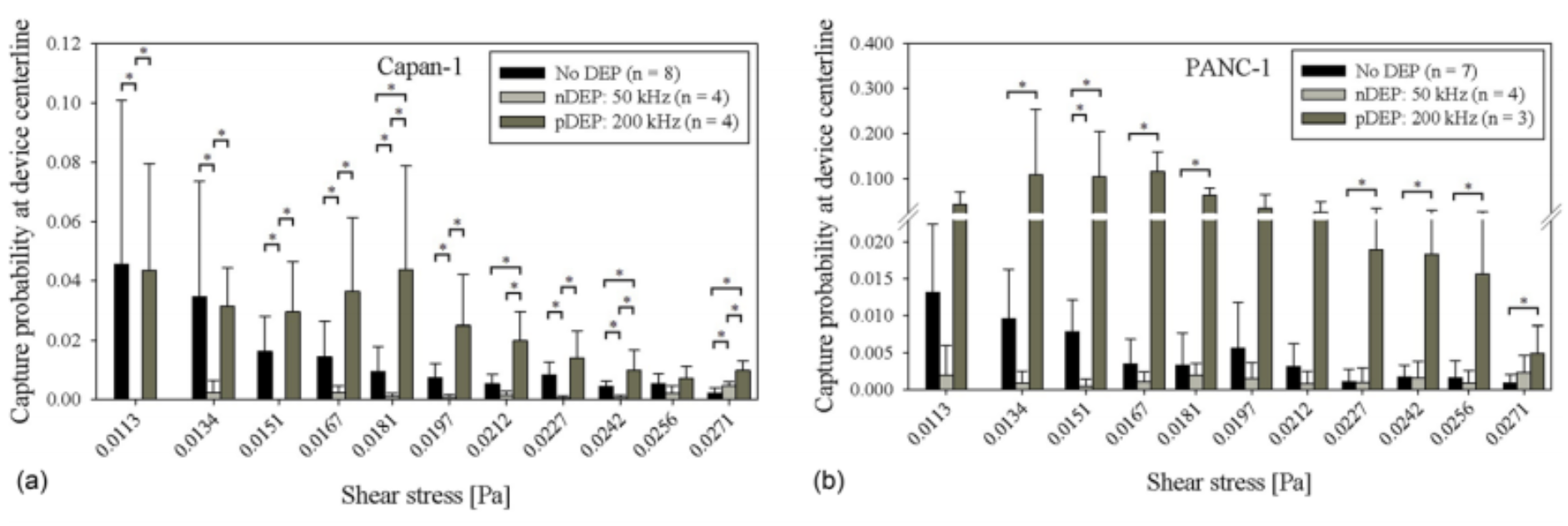
- FIG. 3. Capture probability of Capan-1 cells, 3(a), PANC-1 cells, 3(b), BxPC-3 cells, 3(c), and PBMCs, 3(d), at the central axis of the Hele-Shaw DEP device as a function of shear stress under experimental conditions of no DEP (black bars), 50 kHz (light gray bars), and 200 kHz (dark gray bars). Bars represent the mean capture of the indicated number of experimental replicates (n), and error bars represent standard deviation. A Wilcoxon rank-sum test was used to compare between each pair of DEP conditions, and asterisks (*) indicate significance of differences (P < 0.05).
- FIG. 4. Exponential fits (solid line) to shear-dependent BxPC-3 capture data (symbols) shown in Figure 3(c) for experimental conditions with no DEP, 4(a), nDEP at 50 kHz, 4(b), and pDEP at 200 kHz, 4(c).