Lab on a Chip, 2003: Programmable Modification of Cell Adhesion and Zeta Potential in Silica Microchips
Citation:
Kirby BJ, Wheeler AR, Zare RN, Fruetel JA, Shepodd TJ, Programmable Modification of Cell Adhesion and Zeta Potential in Silica Microchips, Lab Chip 3:5-10 (2003). doi pdf
Abstract:
Spatial patterning of thin polyacrylamide films bonded to self-assembled monolayers on silica microchannels is described as a means for manipulating cell-adhesion and electroosmotic properties in microchips. Streaming potential measurements indicate that the zeta potential is reduced by at least two orders of magnitude at biological pH, and the adhesion of several kinds of cells is reduced by 80-100%. Results are shown for cover slides and in wet-etched silica microchannels. Because the polyacrylamide film is thin and transparent, this film is consistent with optical manipulation of cells and detection of cell contents. The spatial patterning technique is straightforward and has the potential to aid on-chip analysis of single adherent cells.
Figures:
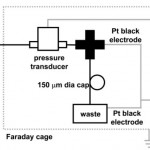
- Fig. 2 Streaming potential measurement apparatus. An HPLC cross-connects the input from the flow-through pressure transducer to waste and allows an electrode to measure the upstream end of the capillary (DAQ: data acquisition system). doi pdf
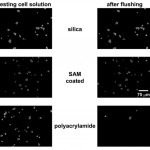
- Fig. 3 Imaging of CATH.a cell adhesion on untreated (bare silica), SAMcoated (acrylate-terminated), and laser-polymerized (polyacrylamide) cover slides. See experimental section for details. Images have been processed (high-pass filter) to highlight cell locations. doi pdf
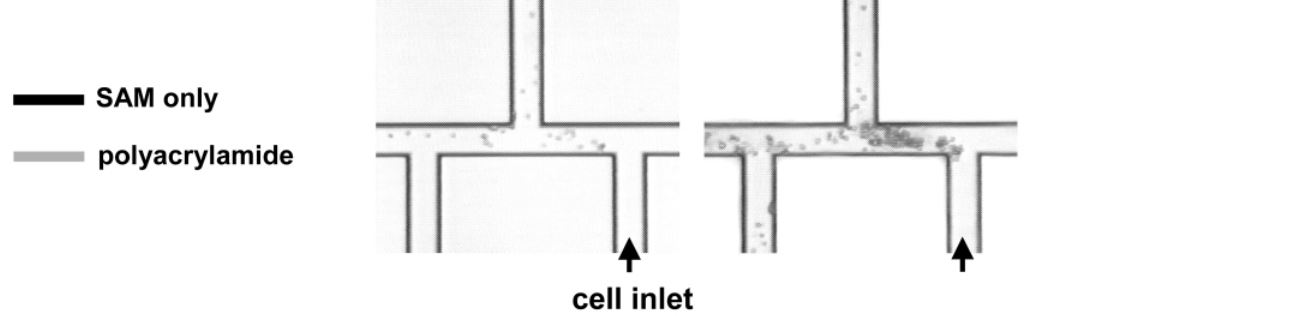
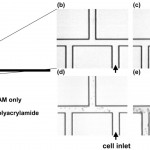
- Fig. 4 Time history of cell adhesion on triple-tee microchip selectively coated with polyacrylamide. All surfaces are coated with the SAM. (a) Shape of microchannel, showing imaged region as well as location of polyacrylamide coating; (b)-(e) sequential time history of cell adhesion. A U937 cell suspension is injected into the port at lower right (labeled cell inlet). All cells visible in images have adhered to wall or are motionless due to clogging–moving cells are completely blurred at this exposure time. Cells adhere and clog all channels without polyacrylamide, but they neither adhere to nor clog polyacrylamidecoated channels. Times of images; (b) t = 0 s; (c) t = 20 s; (d) t = 200 s; (e) t = 900 s. doi pdf
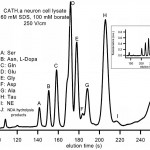
- Fig. 5 MEKC separation of primary amines from a multiple-cell CATH.a neuron cell lysate on glass treated with the SAM. NE: norepinephrine. Inset: separations under the same conditions using bare glass. Separation fidelity and retention time repeatability are similar in the two cases; increases in zeta potential caused by wall-detergent interactions upon coating with SAM lead to reduced elution times in the SAM-coated case. doi pdf