“From the Trenches”- Part 2
What does it mean if my team or a regulatory authority finds a pathogen in my plant?
By Rob Ralyea
Read Part 1 here
It’s Friday afternoon, and you (and your food safety team if you have one) have just put the finishing touches on your Food Safety Plan (FSP). An item of specific concern to you are the requirements for sanitation preventive controls. Sanitation preventive controls are procedures, practices, and processes to ensure that the facility is maintained in a sanitary condition to minimize or prevent hazards such as environmental pathogens, hazards from employees handling food, and food allergen hazards. So the question arises in your mind, how do I do that?
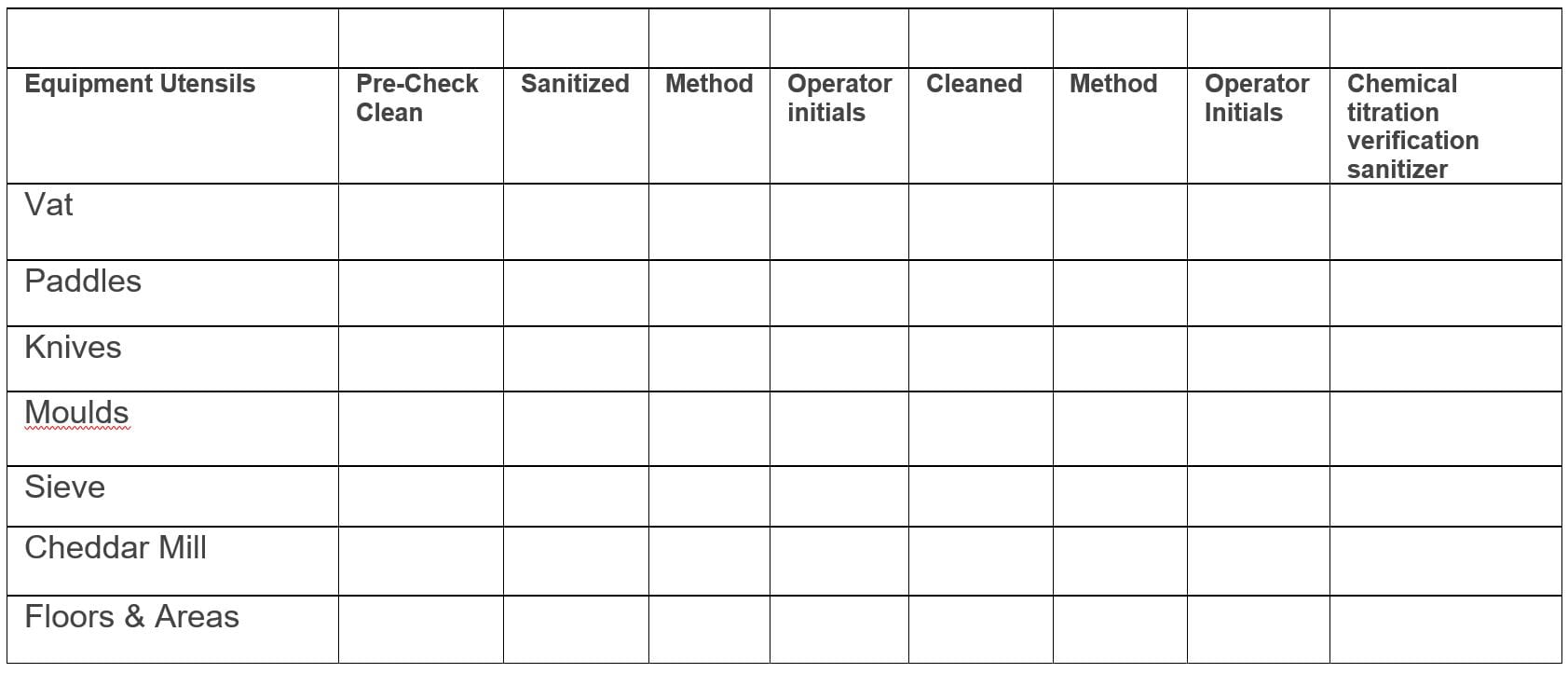
Well, first a cleaning and sanitation record would have to be generated. So, for every piece of equipment, surface, etc, there should be a record of who cleaned what and when. There should also be a record of who verified that it was done and done properly. Here is an example form, but any form that you develop and use must fit your situation.
That brings us to the definition of verification, which are activities required to ensure that preventive controls are consistently implemented and effective in minimizing hazards. Examples of verification activities include scientifically validating process preventive controls to ensure that the control measure is capable of effectively controlling an identified hazard and calibrating (or checking the accuracy of) process monitoring and verification instruments such as thermometers. Verification activities also include reviewing records to ensure that monitoring and corrective actions (if necessary) are being conducted. Verification activities must be documented. Product testing and environmental monitoring are also possible verification activities, required as appropriate to the food, facility, nature of the preventive control, and the role of that control in the facility’s food safety system. Environmental monitoring is required if the contamination of a ready-to-eat food with an environmental pathogen is a hazard the facility identified as requiring a preventive control. So, if you have a sanitation preventive control identified in your FSP, you will need an environmental monitoring program.
The next questions are, what does my pathogen environmental monitoring (PEM) program look like? Where do I sample? How many samples do I need to take? What pathogen am I looking for? Am I only looking for one pathogen or several? There is no ‘book answer’ to any of these questions. They are all plant specific. Your PEM sampling is a complicated task and can’t be completely covered in an extension newsletter article. Volume of samples is generally based on square footage, but if you are a very small producer, going bankrupt via laboratory bills because you are taking voluminous numbers of samples doesn’t make sense either. Truth be told, that could be said for large plants as well. Sampling smart is the best solution which means taking samples that are going to be indicative of the true pathogen content of the environment. Generally speaking, most dairy plants should at a minimum, be looking for Listeria monocytogenes or in some cases Listeria species at a minimum. Some plants may also consider looking for other pathogens like Escherichia coli or Salmonella species, depending on what they might be doing in their plant (if you use ingredients that might bring these into the plant, for example). The very first thing you should do is put in place an SOP that outlines what actions you will take if you find a sampling location that is positive for a pathogen and then follow it when the need arises.
Finding a pathogen in the plant environment is not the end of the world. As a matter of fact, if it’s in the plant, you want to find it and eradicate it before it can spread. As mentioned, you should have a protocol on how to react to positives. If it’s in the environment itself, you don’t have to notify anyone, but you do have to react to the finding. If you do finished product testing or testing of Zone 1 surfaces (Food Contact Surfaces) and have a positive finding, you should notify the regulatory authority. If the product has left your control, you are required to notify the regulatory authority. This brings up the philosophical argument, to do or not to do finished product/Zone 1 testing. First, if your PEM program is working, you might find target organisms sporadically in zone 3 or zone 4 locations. Your goal is to find it and get rid of it before it ever gets to zone1/zone 2 locations. In that case, technically, you should never find a target organism in finished product or in a zone 1 location. If you do, your program is not well designed and is obviously not working. Then again, if you don’t test finished products or zone 1 locations, you will not find positives. Meanwhile, the FDA tests finished products from store shelves randomly, and they have found positives. That is NOT how you want to find out that your PEM program is failing. Having said all of that, the company has to decide whether they want to do finished product/zone 1 testing. Currently there is no legal requirement to do it.
If you want more in-depth training on the topic of Pathogen Environmental Monitoring, there will be a workshop at Cornell University December 5-6. You can enroll here: https://dairyextension.foodscience.cornell.edu/content/1205-0619-pathogen-environmental-monitoring-workshop-0/