Updated January 29, 2020
Project Public Forum, Geneva, N.Y.
Below is a recording of a public forum hosted on August 9, 2017 by Cornell University about the Diamondback Moth project. There are three videos accessible by clicking the upper left of the image.
- Dr. Jan Nyrop of Cornell University explains the need for targeted pest management to ensure the stability and sustainability of the world’s agricultural practices.
- Dr. Tony Shelton of Cornell University gives an overview of the sterile insect technique used to target pest insects, as well as a summary of his 2015 Diamondback Moth research and a description of his planned experiments for 2017.
- Panelists answer audience questions collected both online and in person.
Frequently Asked Questions about the Diamondback Moth project
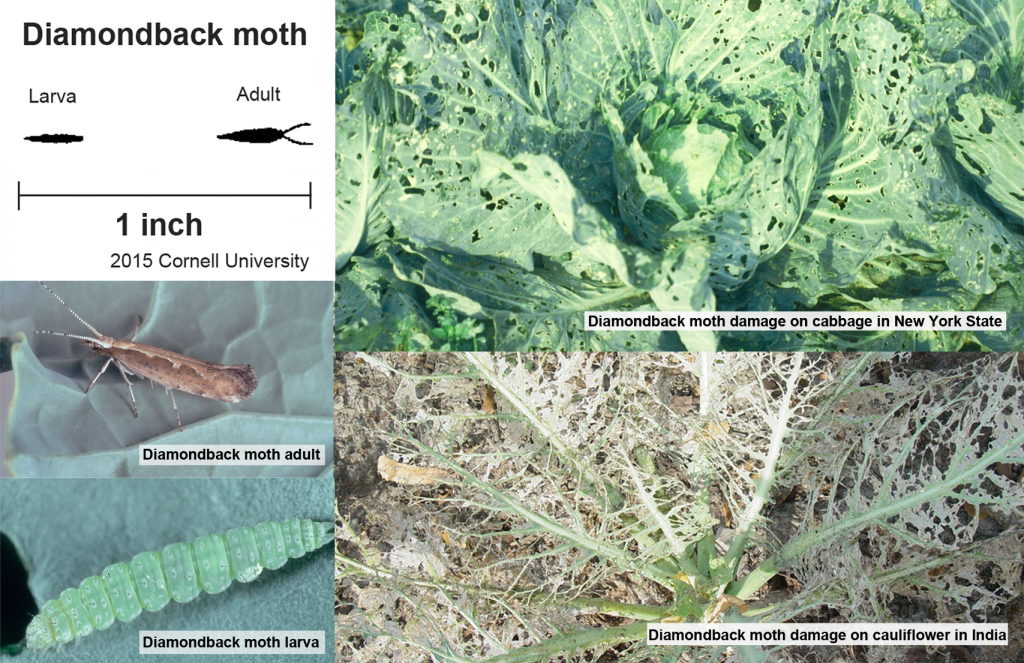
What is diamondback moth (DBM)?
The diamondback moth, Plutella xylostella, is a major agricultural pest and invasive species in New York State, as well as other states and countries. The moths are small, about the length of two grains of rice, but females can lay upwards of 150 eggs during their lifetime, and a generation can be produced in as little as two weeks.
Why are diamondback moths a problem?
The diamondback moth is the world’s worst insect pest of brassica vegetable crops including cabbage, broccoli, cauliflower, kale, radish and many other crops – costing farmers $4-5 billion annually worldwide. The caterpillars chew on leaves and can kill young plants or make the older plant parts unmarketable.
This invasive pest most likely came from Europe, but is now found throughout the world, including New York State and other states that farm brassica vegetables and field crops.
Because of their short generation time and high fecundity, diamondback moth populations can increase rapidly in the field and are not usually sufficiently controlled by natural enemies or other non-insecticidal integrated pest management practices. Hence, New York growers, as well as growers throughout the world, typically spray their crops with insecticides to reduce injury caused by diamondback moth larvae.
Yet this has led to development of resistance to most synthetic and organic insecticides in the diamondback moth, so it is increasingly difficult to control this non-native species and crop damage is increasing worldwide.
There is also concern about pesticide residues on crops, worker safety, and potential hazards to the environment including loss of pollinators and reduced biodiversity.
Alternative insecticide-free methods of control are urgently needed to ensure safe and sustainable brassica crop production in New York and globally.
Who is Oxitec?
Oxitec is a wholly owned subsidiary of Intrexon Corporation with an international team of scientists developing novel ways to control insect pests that spread disease and damage crops. The company is headquartered in Oxford, UK, and uses a ‘self-limiting’ gene developed through modern biotechnology and advanced genetics. This approach leverages the natural reproductive instincts of male insects to find pest females to mate, resulting in reduction of the pest population. For more information on the process of developing self-limiting insects, see the website www.oxitec.com
Is this the first release of a ‘modified’ insect into the environment?
Since the 1950’s there have been billions of ‘modified’ insects released into the environment as a tool for controlling insect pests. The largest of these programs involves the American screwworm, a parasite of all warm-blooded animals including livestock, wildlife, pets, zoo animals and occasionally humans. Females lay their eggs in open wounds and their maggots feed on the flesh and debilitate animals. This USDA project uses radiation to sterilize male screwworms and then releases them en masse to mate with females who then can no longer reproduce, hence the term sterile insect technique (SIT). This technology has been used to eradicate the screwworm from large areas in the US and Latin America and was praised in Rachel Carson’s 1962 book, Silent Spring. However, sterilization by radiation has many drawbacks and therefore has only been used successfully on a few insect species.
Using genetic engineering (GE) overcomes many of these drawbacks. In the southern US, the pink bollworm, a pest of cotton, was genetically engineered to express a fluorescent protein for accurate field monitoring in an existing SIT program. In the first field trials of a GE insect in the United States, more than 20 million radiation-sterilized moths of this strain were released from 2006 until 2015 as part of the pink bollworm management program in Arizona.
Besides genetic engineering being used for monitoring insects in the field, it has also been used for more direct controlling of an insect pest. Mosquitoes have been engineered to express a self-limiting trait and a fluorescent protein. In Brazil over 150 million self-limiting GE male mosquitoes have been released since 2015 for control of Aedes aegypti, the mosquito species that transmits the pathogens causing dengue fever, the Zika, Chikungunya and yellow fever. Previous trials in Brazil, Panama and the Cayman Islands have demonstrated over 90% reduction of wild Aedes populations, for a total global release of more than 200 million mosquitoes. These trials have been published in peer-reviewed journals (see below).
The diamondback moth project has been the first open field release of a genetically engineered self-limiting insect in North America. But, in very real sense, it is simply an improved version of a program for modifying an insect for pest control that began in the 1950s, and continues to this day.
Is there successfully completed diamondback moth research?
Yes, research on the Oxitec GE diamondback moth has proceeded in a stepwise manner where laboratory and greenhouse studies have been completed in the United Kingdom and United States prior to consideration of outdoor studies as the next logical step. The results demonstrated that the GE diamondback moth performed as expected and successfully reduced the population of the pest moths. An enclosed field cage study was conducted in the summer of 2015 at the Cornell New York State Agricultural Experiment Station in Geneva, NY. This experiment evaluated the performance of the moths under natural conditions inside the cages. The results indicated that the GE diamondback moths are able to perform well in field cage conditions, offering promise for future diamondback moth management. Based on an independent review of the 2015 data from the caged study, the team at Cornell determined that a small-scale field trial was the next logical step in the evaluation of the self-limiting diamondback moth approach.
What happened during the field study of 2017?
The first limited field trials were held in Geneva, NY, between August and September of 2017. These comprised of small-scale field releases for scientific evaluation of the field biology of the GE diamondback moth, including their movement and lifespan in a trial cabbage field. A total of 11 releases were made using a total of 16,500 GE moths. As expected, no GE moths were found outside of the field in which they were released. Since late 2017, our scientists have been continuing to analyze the data for recapture of the released moths, distance traveled from the release site, moth longevity in the field, and other factors. Our paper with the results of our study was published by Frontiers in Bioengineering and Biotechnology in January 2020.
Was the field study under regulatory control?
The Cornell University College of Agriculture and Life Sciences is committed to ensuring that the trial is conducted in compliance with all applicable Federal and State regulations, as well as internal University requirements for oversight on Biosafety. As part of the regulatory process, the US Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) Biotechnology Regulatory Services (BRS), prescribes specific conditions which must be adhered to in order to ensure the trial is conducted in compliance with federal law. Compliance verification activities are a routine part of the regulatory oversight activities of the BRS, and Oxitec and Cornell work together to ensure all activities are carried out consistent with prescribed conditions. On April 19, 2017, USDA’s Animal and Plant Health Inspection Service (APHIS) announced it would make available for public comment a draft Environmental Assessment (EA) prepared in connection with the permit application for the field release of genetically engineered (GE) diamondback moths. The draft EA was available for a 30-day public review and comment period, which ended on May 19, 2017. On July 7, 2017, notice of the availability of a final Environmental Assessment and Finding Of No Significant Impact (FONSI), along with the issuance of a permit for the field release, was published in the Federal Register. Cornell University’s Institutional Biosafety Committee and the College of Agriculture and Life Sciences gave their approvals to the project.
The 2015 field cage trials were conducted under USDA-APHIS-BRS permit number 13-297-102r, which was issued following extensive evaluation of the proposed study by the USDA-APHIS-BRS regulators. This evaluation process, which involved a public comment period, concluded in publication of an Environmental Assessment (EA) and a Finding Of No Significant Impact (FONSI).
What about safety?
Human health considerations form a key part of the regulatory process that APHIS-USDA-BRS undertakes in evaluating any trial application. The proteins from the fluorescent marker gene and the self-limiting gene are the same across the Oxitec portfolio of self-limiting insect control products. Independent evaluation by the Food Allergy Research and Resource Program of the University of Nebraska–Lincoln of the toxic or allergenic potential of these proteins, known as DsRed2 (fluorescent marker) and tTAV (self-limiting gene) has been key to regulatory submissions for all Oxitec products to date. No toxic or allergenic potential has been identified. Additionally, the impacts on non-target organisms through oral exposure of GE insects carrying the DsRed2 and tTAV proteins have been evaluated through direct feeding studies, consistent with international guidelines, involving predatory insects as well as guppy fish, and no adverse impacts were observed even when fed an artificially high diet of 100% GE insects (e.g. Nordin et al. 2013).
How does the self-limiting approach work?
Oxitec male moths pass on a self-limiting gene that prevents their female offspring from reaching adulthood. This reduces the number of females able to reproduce, and so with repeated releases of Oxitec’s male moths, the pest population in the release area shrinks. This method is self-limiting and does not persist in the environment, unlike gene drive approaches, which are designed to persist in the environment long term.
What about environmental impacts?
Unlike conventional insecticides that can have off-target effects, the self-limiting approach by mating is species-specific, impacting only the intended pest diamondback moth population. This is because the released GE diamondback moths will only mate with their own species, producing offspring which carry the self-limiting gene.
The open field trials were limited to the area of the test crucifer fields at our research station and, based on previous studies, these insects were unlikely to travel far from the ample food available to them at the test field. Adult diamondback moths have a short life span in the field, typically living for less than a week. Data available from tests of the proteins produced by the GE diamondback moths for toxicity and allergens show they are non-toxic and non-allergenic. Oxitec has also conducted or commissioned feeding studies in a variety of non-target organisms such as spiders, rove beetles, fish and parasitoids, all using insects carrying the same introduced genes as the GE diamondback moths as the feed source. In all cases no adverse effects were observed. Thus, if predators–or even people–ingest a modified moth, it would be no different than ingesting a pest diamondback moth. The test field will be insecticide-treated after the study, and the moths and the crop will be destroyed and will not enter the food or feed chain.
The potential environmental consequences of the GE diamondback moth release are rigorously evaluated as part of the USDA application process. In a previous application for the GE diamondback moth release, under which the 2015 caged trials were conducted, the USDA issued a comprehensive “Finding of No Significant Impact” statement which concluded that the release should be permitted. The decision was based on a rigorous evaluation of environmental considerations including plant communities, wildlife and biodiversity, among other elements.
Why is tetracycline used to produce Oxitec insects?
Questions have arisen regarding the use of tetracycline for producing Oxitec moths and whether this presents a risk to the environment because of tetracycline resistance. Tetracycline is a common antibiotic used in livestock farming.
Most all organisms contain bacteria and there is a concern that such bacteria may have been exposed to tetracycline and developed resistance to it, and that such resistant bacteria could transfer to other organisms. In the case of Oxitec moths, only male Oxitec moths will be released and they, and any bacteria they carry, were not exposed to tetracycline while they were in the lab so would not have evolved resistance during this time. So the likelihood of Oxitec moths contributing to tetracycline resistance in the field would be similar to that of a non-Oxitec moth. Furthermore, it should be noted that tetracycline resistance occurs naturally in many soil-dwelling bacteria.
Oxitec insects carry a self-limiting gene, which incorporates a genetic switch that is turned off in the presence of tetracycline, or suitable analogues. In the lab, tetracycline is used to suppress the self-limiting gene, allowing female Oxitec moths to be produced so the colony can develop and produce the next generation, but females and any bacteria they carry are not released. In the lab tetracycline simply allows Oxitec females to survive to adulthood (male counterparts do not require tetracycline to reach adulthood). Tetracycline (Tet)-controlled genetic switches are some of the most common switches used in biology and have been used for decades.
The potential for antibiotic resistance in the case of rearing Oxitec mosquitoes was examined by the Food and Drug Administration (FDA)-led team in their review of a proposed trial in the Florida Keys. After exhaustive review, they concluded that “the likelihood of the adverse effects associated with development of anti-microbial resistance is extremely low and the risk is negligible”. In addition, the US Department of Agriculture’s Finding Of No Significant Impact for the Oxitec diamondback moth studies assessed this topic, and concludes by saying, “For the one-year study proposed, the risk to human health as result of fostering the development of tetracycline resistance in bacteria is considered negligible.”
Can organic growers use this technology?
Although organic growers use fertilizers and pesticides, they are prohibited from using any product that is synthesized, including fertilizers and pesticides. Therefore, they cannot utilize GE insects.
Who benefits from this research?
Using GE diamondback moths provides a means of species-specific control over this invasive pest, thereby reducing the crop damage caused by diamondback moth larvae. Currently, New York growers, as well as growers throughout the world, typically spray their crops with insecticides to control diamondback moth (Talekar & Shelton 1993). However, this has led to the insect developing resistance to most insecticides and increased levels of crop damage, as well as increased concerns about worker safety, pesticide residues on crops, and potential hazards to the environment. By controlling diamondback moth and decreasing reliance on insecticides, new technologies such as the GE diamondback moth will bring local and global benefits from improved food security, food safety, and environmental protection.
Insects are becoming increasingly important to control for both the benefit of agriculture and human health, and there is a pressing need for new technologies to be developed. Growers in New York and worldwide are faced with an expanding list of new invasive pests (e.g. spotted wing Drosophila) for which novel and safer technologies, including genetic engineering, are needed. Scientists and administrators at Cornell are poised to be leaders in ensuring research on these new technologies is conducted in a safe, transparent and properly regulated matter.
Who funds this work?
Oxitec, the company that developed GE DBM, is paying the cost to conduct this research project. However, as with all outside-funded projects at Cornell, Oxitec has no influence over the outcome of the trials or publication of results. This is clearly stated in Cornell’s Faculty Handbook. Neither Professor Shelton nor anyone in his program will receive financial benefits from this work.
Where can I get additional information?
- Cornell site: http://shelton.entomology.cornell.edu
- Oxitec site: www.oxitec.com/dbm and email: dbmtrial@oxitec.com
- Final Environmental Assessment issued by USDA as part of permit for 2015 trials (http://www.aphis.usda.gov/brs/aphisdocs/13_297102r_fea.pdf)
- Finding Of No Significant Impact issued by USDA as part of permit for 2015 trials (http://www.aphis.usda.gov/brs/aphisdocs/13_297102r_fonsi.pdf)
- Nordin et al. 2013. Oral ingestion of transgenic RIDL Ae. aegypti larvae has no negative effect on two predator Toxorhynchites species. PLoS One 8: e58805 http://www.ncbi.nlm.nih.gov/pubmed/23527029
- More information on diamondback moth can be seen at http://web.entomology.cornell.edu/shelton/diamondback-moth/index.html
- Carvalho et al. (2015). Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis 9(7) DOI.org/10.1371/journal.pntd.0003864
- Harris et al. (2011). Field performance of engineered male mosquitoes. Nature Biotechnology, 29, 1034-1037. DOI 10.1038/nbt.2019
- Harris et al. (2012). Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nature Biotechnology, 30, 828-830. DOI 10.1038/nbt.2350
- Gorman et al. (2015). Short-term suppression of Aedes aegypti using genetic control does not facilitate Aedes albopictus. Pest Management Science, 72, 618-628. DOI 10.1002/ps.4151
- Talekar, N.S. & Shelton, A.M. 1993. Biology, ecology and management of the diamondback moth. Annual Reviews of Entomology, 38, 275-301.
- Sterile Insect Techniques: Principles and Practices in Area-Wide Integrated Pest Management (2005). V.A. Dyck, J. Henrichs and A.S. Robinson. Springer. http://www-naweb.iaea.org/Nafa/ipc/public/Sterile_Insect_Technique_book.pdf#page=14