  |
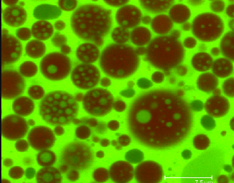
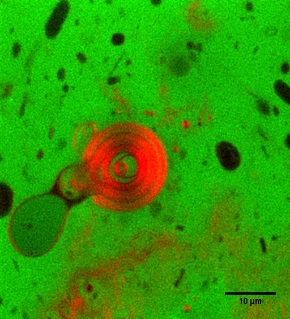
Liposomes are intelligently designed nanoscale vesicles that use a phospholipid bilayer as a protective barrier, similar to the natural storage, transport and secretory vesicles generated by living cells. Because of their amphiphilic nature, they are able to simultaneously encapsulate both hydrophilic and hydrophobic compounds, giving flexibility to deliver many types of cargo. They are particularly ideal for site specific delivery of bioactives to the body, due to their similarity to natural cells. Used as a biocompatible carrier system, liposomes can also promote the physiological activity and safety of the entrapped components. They are already being used in the pharmaceutical industry, but their reliance on the use of organic solvents and lack of control of particle formation limits their application in food and nutrition systems. Taking advantage of high pressure, we have designed and developed a unique venturi-based system for the production of liposomes using supercritical carbon dioxide, a benign alternative to conventional solvents. In this system, nanoliposomes are produced using separate atomized streams for the cargo and carrier, and as such greater control of the final product size and lamellarity is achieved. Not only will this system allow for the safe production of these nanoliposomes, but also for the study of the fundamental aspects of nanoparticle formation. Current focus includes quantifying the effects of atomization mechanics on the formation of liposomes and development of models for the prediction of liposomal formation. Efficacy of the process is aimed to be demonstrated by the production of nanoliposomes containing selected bioactives and subsequent study of their storage stability and modeling of release kinetics. |
