Below is a report done by Andrew Harrison while at Harper Adams University. Mr. Harrison was an exchange student at Cornell University, enrolled in the Applied Dairy Cattle Genetics course which developed this website. Upon returning to Harper Adams University, Mr. Harrison compiled this report, building upon the foundation of material learned within our course. He developed the page “Bull Selection Based on Economic Values for Genomically Tested Herds” under Economic Impact.
An evaluation of applying genomic information to the females in a dairy herd (2 page leaflet)
Summary
The aim of this report is to evaluate the application of genomic information to the females in a dairy herd. The concept of genomic selection has been in existence for over forty years, however only recently has technology advanced sufficiently to map the bovine genome and compute the information this provides. Genomic testing challenges the traditional method of progeny testing, which can take 5-6 years and improves genetic progress through increasing the accuracy of selection, increasing the selection intensity and reducing the generation interval. The advancement of genomic evaluations has led to the manufacture of low cost, low density Single Nucleotide Polymorphism (SNP) chips, to be used for the genomic evaluation of females in commercial herds for improved selection and management.
Genomic evaluations have been shown to be an accurate predictor of future performance, superior to sire Predicted Transmitting Ability (PTA) alone. Genomic evaluations improve the accuracy of selecting replacements, marketing pedigree heifers, identifying incorrect parentage which will improve mating plans and avoid breeding carriers of deleterious recessive alleles. Genomic evaluations are likely to be applied to beef cattle in the future, however there are difficulties regarding the lack of large reference populations of animals of similar genetics.
The economics of applying genomic information to the females in a dairy herd will vary between individual farms. As a general rule, farms with no pedigree information, selecting a proportion of total heifers as replacements and using the genomic evaluation information for a number of decisions will have the greatest return on investment.
- Introduction
Progeny testing has been the foundation for genetic improvement in dairy cattle for over half a century and in combination with Artificial Insemination (AI) has led to rapid genetic gains. However, progeny testing, a process which can take 5-6 years, results in a long generation interval, which slows genetic progress (Pryce and Daetwyler, 2012). Progeny testing is also ineffective at selection for traits, such as feed efficiency, that are difficult and expensive to measure (Weigel et al., 2015).
Genomic evaluations use information about Single Nucleotide Polymorphisms (SNPs) in a genotyped reference population with phenotypic data to estimate the markers effects. The effects of the markers predicted in the reference population, combined with any polygenic effects, are then used to estimate the breeding value of individuals in the general population with no offspring or parentage information (Thomasen et al., 2012; Weigel et al., 2015). The inclusion of polygenic information allows selection of low-frequency Quantitative Trait Loci (QTL) that may not be detected by the markers (Hayes et al., 2009).
Most producers will associate genomics with genomic bull proofs. Genomics has revolutionized the dairy genetics industry and has the potential to reduce bull progeny testing costs by 92% (Schaeffer, 2006) through moving to an entirely genomics based proving system. French breeding companies have stopped official progeny testing (Pryce and Daetwyler, 2012) and all bulls in the USA are selected on the results of genomic testing (Weigel et al., 2015).
Using genomics for bull selection has the potential to increase genetic gain. The breeder’s equation determines the level of genetic gain per year. (Willis, 1998)
Assuming that genetic variation is constant, genomic selection has the potential to increase the accuracy of selection through improving reliability of unproven sires compared to parent average (60% vs 30%, VanRaden et al., 2013), selection intensity through a wider screening of young bulls, whilst decreasing the generation interval by removing the need to progeny test young bulls.
As the cost of genomic testing reduces, it becomes feasible to test the female members of the herd (Pryce and Daetwyler, 2012). In Canada in 2006, a 10K SNP chip would cost approximately £200 (Schaeffer, 2006) whereas a 10K SNP chip including genomic evaluation is currently available for British dairy farmers via NMR at a cost of £32 plus VAT (NMR, 2014). The aim of this report is to evaluate the different methods of applying genomic technology to the females in a dairy herd and identify potential future uses of genomics.
2.0 A brief history of genomics
The concept of genomic selection is not a recent phenomenon. Smith (1967), identified the possibility of selection through known loci on the genome and accurately described many of the benefits of genomic technology. This included, parentage testing, selection for traits with poor heritability and the ability to reduce the generation interval. However, it took forty years for technology to advance to be able to sequence the bovine genome and identify the quantitative trait loci (QTL) which contribute to the variation in a trait in the form of SNP (Hayes et al., 2009; Elsik et al., 2009). At the same time the cost of genotyping has significantly reduced.
Genomic evaluations have only recently developed. The first genotype ‘chip’ for cattle was released in December 2007 (Wiggans, 2011). ‘Chips’ produce the genotype from which the genomic evaluation is calculated. The first official genomic evaluations began in January 2009 in the USA and in 2013 in the UK (Wiggans, 2011; Holstein UK, 2013).
3.0 Demonstrating the relationship between genomic evaluations and future performance
To be able to justify investment in genomic testing, it is essential to prove that genomic evaluations are an accurate representation of future performance.
Weigel et al., (2015) compared the genomic Predicted Transmitting Ability (PTA) for a number of traits, to the PTA of each heifer’s sire on subsequent performance. Figure 1 demonstrates the relationship between first lactation milk yield and heifers sorted by sire’s PTA Milk, or the heifer’s own genomic PTA Milk.
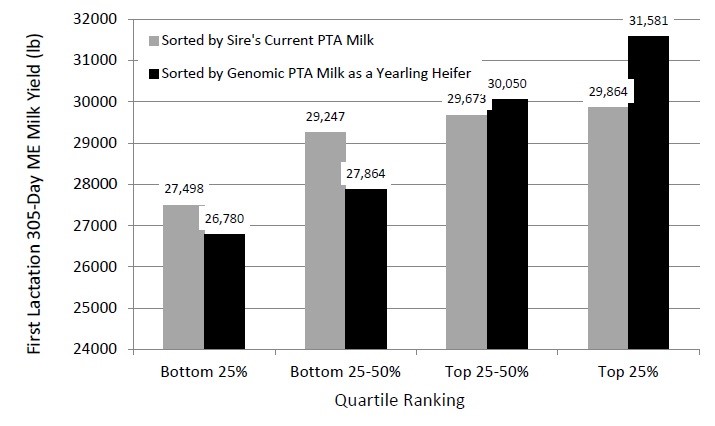 Figure 1: Average first lactation ME 305-day milk yield for 411 Holstein cows in the Allenstein Dairy Herd at UW-Madison, according to quartile for genomic PTA milk at 12 months of age and quartile for sire’s current PTA for milk yield (Source: Weigel et al., 2015)
Figure 1: Average first lactation ME 305-day milk yield for 411 Holstein cows in the Allenstein Dairy Herd at UW-Madison, according to quartile for genomic PTA milk at 12 months of age and quartile for sire’s current PTA for milk yield (Source: Weigel et al., 2015)
As described in Figure 1, the difference between top and bottom quartiles for cows sorted by sire PTA Milk was 2,366 pounds, whereas when sorted by genomic PTA Milk, the difference was 4,801 pounds. This shows that genomic data is much more accurate than sire information for predicting future milk production and that genomic PTA milk is a reliable measure of future milk production.
The heritability (h2) of a trait is the proportion of the phenotype which is attributed to the genetics of the animal, not the environment (Willis, 1998). In high heritability traits, information from each daughter from progeny testing is worth more than for low heritability traits. For example, for the same level of reliability as genomic PTA Milk, for PTA Milk (h2=0.3), phenotypic data would be required from 16 daughters. For PTA Daughter Pregnancy Rate (DPR) (h2=0.04), it would require 67 daughters to provide the same reliability as genomic PTA DPR (VanRaden et al., 2009). Genomics therefore has the potential to provide greater genetic gain in low heritability traits than high heritability traits.
To demonstrate this, Figure 2 shows days open in the first lactation compared with heifers sorted by sire’s PTA DPR and genomic PTA DPR.
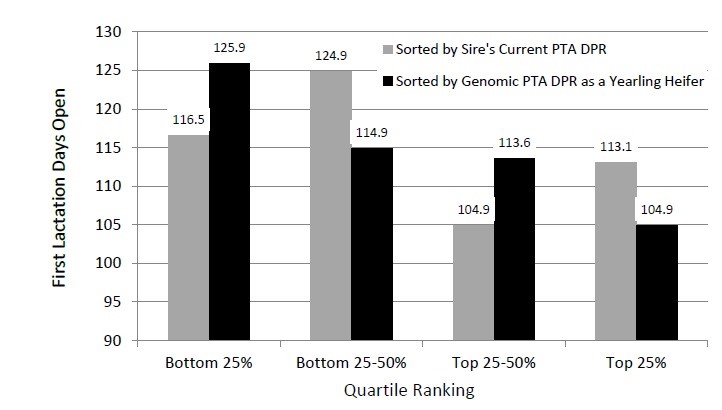 Figure 2: Average days open in first lactation for 240 Holstein cows in the Allenstein Dairy Herd at UW-Madison, according to quartile for genomic PTA for daughter pregnancy rate at 12 months of age and quartile for sire’s current PTA for daughter pregnancy rate. (Source: Weigel et al., 2015)
Figure 2: Average days open in first lactation for 240 Holstein cows in the Allenstein Dairy Herd at UW-Madison, according to quartile for genomic PTA for daughter pregnancy rate at 12 months of age and quartile for sire’s current PTA for daughter pregnancy rate. (Source: Weigel et al., 2015)
Figure 2 identifies that there is no strong correlation between sire’s PTA DPR and days open in the first lactation. This is unsurprising due to the low heritability of DPR (h2=0.04) as fertility mainly influenced by environmental factors (Marti and Funk, 1994). However, there is a clear relationship between genomic PTA DPR and first lactation days open. This suggests that, unlike traditional selection methods, genomic technology can lead to rapid genetic gain in traits with low heritability.
4.0 Methods of applying genomic evaluation to a herd
As low density SNP ‘chips’ have become available, the cost of genomic evaluation has substantially reduced. With genomic testing for females now costing £32 (NMR, 2014), there is potential for farmers to genotype the female members of the herd. How can this information be applied to improve herd management?
4.1 Selecting replacements
The widespread use of sexed semen has led to an increase in the production of females and improvements in management has led to reduced culling rates (Weigel et al., 2012). This has led to an excess of heifers on many dairy farms. Genomic testing has the potential to sort replacement heifers to a higher level of accuracy than traditional methods such as Parent Average. Inferior animals can then be removed as parents of the next generation, either sold as a calf, later in life, inseminated with beef semen or as recipient animals for embryo transfer (Weigel et al., 2012).
It has been shown that genomic selection is economical for most situations on commercial farms (Weigel et al., 2012), only two more heifers are required to be available than necessary for replacement for genomic selection to be economic (Calus et al., 2015). However there are many factors that affect the degree of benefit to dairy farms, including the cost of genotyping, the economic value of the genetic gain, the gain in the accuracy of selection, the replacement rate and the number of available heifers (Calus et al., 2015).
The use of sexed semen increases the genetic gain of using genomic selection, as it increases the number of heifers and so increases the selection intensity. However, the greater the selection intensity, the greater the pro rata genotyping costs if all animals are genotyped. Weigel et al., (2012), describes selecting the top 30, 20, or 10% of heifer calves with known sires. Expected gains in lifetime net merit due to genomic testing were $169, $206 and $254 respectively, whilst the prorated cost of genotyping was $81, $121 and $242.
The greater the amount of pedigree information the lower the economic return of genomic selection. This is because when no pedigree information is available, genomic testing offers a greater increase in reliability in comparison to sire PTA and PA respectively (Weigel et al., 2012). It is rare that no sire information is available, only in farms using natural service from a team of bulls would this be possible.
If a producer is only genotyping to make one decision, i.e. culling the bottom 20%, selecting the top 5% for embryo transfer and the sire is known or pedigree information is available, pre-sorting the heifers by sire PTA or PA is often more cost effective (Weigel et al., 2012; Calus et al., 2015). Pre-sorting of the bottom 50% and selective genotyping led to greater gains than selective genotyping of the middle or top 50% if removing the bottom 10-40%. This is because when testing the middle or top 50% sorted by PA, low breeding value heifers are identified and included in a group which contains high breeding value heifers for which PA was an underestimate, but were never identified. Conversely, genotyping the top 50% leads to greater gains when selecting for the top 10-40% (Weigel et al., 2012).
Genotyping all animals becomes more efficient when many selection decisions are made. For example, a common strategy would be to use genomic testing to identify the top 10% of heifers for embryo transfer on a £PLI basis, the 10th to 50th percentile for sexed semen insemination, the 50th to 75th percentile for conventional semen insemination and to remove the genetics of the 75th to 100th percentile from the herd. When a number of decisions are being made across the whole range of genetic value it is no longer worthwhile to pre-select animals for genomic testing.
4.2 Marketing pedigree heifers
Genomic selection, unlike PA requires no previous pedigree information and so can be applied to commercial dairy farms who have previously had no experience in pedigree breeding. This opens up the opportunity to identify valuable females with different pedigrees to traditional breeders, and provide additional income to farms which have relied on milk production as the major source of income (Weigel et al., 2012).
A heifer that has been genomically tested has breeding values with similar reliability to a cow with three or four lactations, as well as superior information regarding traits with low heritability, such as fertility and lameness (h2 = 0.04 and 0.02 respectively; Pritchard et al., 2013). Genomic testing is less biased than that derived from pedigree testing (Pryce and Hayes, 2012). This should see a premium in pedigree sale prices for genomically tested animals, which will eventually become the norm. This depends on the quality of the genomic evaluation, a poor genetic evaluation would have a negative impact on sale price. It is therefore recommended if the farmer intends to sell pedigree heifers, to preselect with PA those animals which are likely to have good genomic evaluations.
4.3 Parentage Verification
There is little published information regarding the rate of parentage misidentification on dairy farms. It would appear that there is a high level of variation between farms. Herds that block calve are likely to have higher levels of misidentification as there are many calves being born over a short period of time (Pryce and Hayes, 2012). Banos et al., (2001) estimated 11% and Weigel et al., (2012) assumed 15%. Gelderman et al., (1986) found a misidentification rate of 13.2% in Germany, however previous studies in Germany varied between 4 and 23%.
A theoretical paternity error of 11% was added to the pedigrees of US Holstein cows (Banos et al., 2001) which led to a decrease in inbreeding. This suggests that farmers are passively selecting for increased inbreeding, by focusing on a few high genetic merit sires.
Genomic evaluations are able to verify the paternity of calves. If the dam has been genotyped, the calf can also be assigned to the correct dam (Pryce and Hayes, 2012). This will improve the accuracy of pedigrees.
4.4 Mating Plans
Inbreeding impacts profitability by affecting fitness and production traits (Smith et al., 1998). Mating plans constrain the level of inbreeding by predicting potential progeny inbreeding by constructing relationships from pedigree information (Kinghorn, 2011). The benefit of controlling inbreeding far exceeds the penalty in reduced genetic gain in the next generation (Pryce et al., 2012).
Parentage verification via genomic testing will allow for greater accuracy of mate allocation in mating plans. This should further control the level of inbreeding in dairy cows (Pryce and Hayes, 2012). Using genomics, the proportion of the genome that is identical by descent (IBD) can be calculated and genetic gain is increased compared to pedigree relationships (VanRaden, 2008). The rate of genomic inbreeding is 3 times higher when using pedigree mating plans compared to genomic mating plans (Pryce et al., 2012). Genomic mating plans result in an evenly distributed increase in IBD across the genome, whereas pedigree matings result in greater increases in inbreeding at QTL (Pryce et al., 2012). The similarity between genomic and pedigree mating plans increases with the number of generations of pedigree information. Therefore there is a greater potential reduction in inbreeding for farms with little or no pedigree information (Pryce et al., 2012).
4.5 Avoiding deleterious recessive alleles
Genomic evaluations test for deleterious recessive alleles as part of the evaluation. In the UK, genomic evaluations automatically identify carriers for fertility haplotypes, Deficiency of Uridine Monophosphate Synthase (DUMPS) and Bovine Leukocyte Adhesion Deficiency (BLAD). For an additional charge, Complex Vertebral Malformation (CVM), Brachyspina, Beta Casein A2, Coat color and indirect polled test (Holstein UK, 2013).
Genomics have allowed the discovery of lethal recessives, as haplotypes were identified with a high population frequency but were never found in the homozygous form. As no phenotypic data exists, defects that cause embryo loss are very difficult to detect (VanRaden and Miller, 2006).
There have been 10 haplotypes identified that are detrimental to fertility, one in Ayrshires (AH1), two in Brown Swiss (BH1, BH2), five in Holsteins (HH1 to HH5) and two in Jerseys (JH1, JH2) (Cole et al., 2015). When identified, carrier frequencies for HH1, HH2, HH3, JH1 and BS1 ranged from 2 to 21% in genotypes animals (VanRaden et al., 2011). The identification of these animals through genomic evaluation will ensure carrier females will not be bred with a carrier bull.
Selection against carrier sires will only slightly improve future fertility. A carrier bull will reduce average conception rate by carrier frequency divided by 4 (VanRaden et al., 2011). For example, if average conception rate is 40% and the carrier frequency is 4%, the average loss in conception rate is 40%(0.04)/4=0.4 percentage points. This is relatively insignificant compared to the variation in conception rate traits between sires, and between herds.
5.0 Potential future uses of genomics
Genomic evaluations are still a new phenomenon in dairy cattle breeding. Genomics began in dairy cattle as there is extensive historical pedigree information, widespread use of AI sires, progeny testing programs, high value animals and a long generation interval which can be reduced by genomics (Wiggans, 2011). Given these factors, genomic technology will likely be implemented in beef cattle breeds in the future. Research has been conducted into the Limousin (Todd et al., 2014) and Charolais (Gunia et al., 2014), with the aim of reducing the cost of progeny testing. The accuracy of genomic estimated breeding values (GEBV) in beef cattle is expected to be less than in the Holstein breed even for the same reference population size. The lower accuracy of bull EBV is due to a low use of artificial insemination (AI) compared to dairy breeds (Gunia et al., 2014).
Reliability of genomic traits is substantially lower in beef cattle than dairy cattle. Gunia et al., (2014) identified accuracy of genomic predictions of 0.42, 0.34, 0.45, 0.52 and 0.27 for birth weight, calving ease, weaning weight, muscular development and skeletal development respectively. As reliability is accuracy squared (Hansen, 2014), this results in reliabilities of 0.17, 0.11, 0.20, 0.27 and 0.07 respectively. These results are lower than the parent average reliabilities of the Holstein breed (0.30) (VanRaden et al., 2013). This highlights the impact that AI has had on genetic improvement in the Holstein breed. However, if a large enough reference population of one breed can be identified (Swan et al., 2012) to create a reliable genomic evaluation, genetic gain is likely to be larger than in Holsteins. This is because the fewer generations of pedigree information, the greater the impact of genomics on genetic gain (Pryce et al., 2012).
6.0 Economic analysis of applying genomic evaluations to a herd
This report has outlined the many benefits of applying genomic evaluations to the females in a herd of dairy cows. However, is there sufficient benefit from the increase in accuracy and reliability over sire PTA and PA to justify the cost of the evaluation?
Utilizing the data from Figure 1 (Weigel, 2015), the difference in first lactation 305-day ME milk yield between the top 75% of cows based on genomic PTA and sire PTA is 237 pounds per lactation (107.5 kg). If this gain in production is replicated across the cows lifetime (3.2 lactations; Holstein UK, 2015), this equates to an increased lifetime production of 344 kg. Applying an average Margin over Purchased Feed (MOPF) of 17.15ppl in September 2015 (AHDB Dairy, 2015), results in an increase in lifetime revenue of £59. Given that a low density genomic evaluation costs £32 per animal (NMR, 2014), the increased profitability of each animal is £27. Given an average herd size of 133 cows in the UK in 2014 (AHDB Dairy, 2015b), this leads to a net profit of £3591.
In practice the gain would be greater, as there would be an increase in genetic gain, so future generations would be more profitable. The method of selection will affect the increase in profitability, this simple scenario presumes heifers are selected purely on genomic PTA milk, whereas in reality heifers would be selected on an index, such as NM$, or £PLI, or a combination of individual traits. This would contribute to improving profitability over a selection of traits, such as improved PTA DPR which would increase fertility, reduced mastitis through improved PTA SCC (Somatic Cell Count) and improved longevity through PTA PL (Productive Life).
There will be variation between farms, a farm with a poor SCC will benefit greater economically if genomic PTA SCC is used to move up a milk quality band, or reduce the number of mastitis cases, compared to a herd that already has a low average SCC. The availability of pedigree data is important, as PA is more reliable than sire’s PTA as used in the example, so there is likely to be a smaller economic gain.
7.0 Conclusions
Genomic evaluations have progressed rapidly over the past decade. As genomic evaluations have become more widespread, reliability has increased and the cost of evaluations has decreased. The release of low density SNP ‘chips’ for genomic evaluations, at a lower cost than traditional high density ‘chips’ has made it economical to test female members of the herd to identify each animal’s genetic value. Genomic PTA’s has been shown to be an accurate representation of future performance and more reliable than sire PTA or Parent Average. The greatest benefit to be found from genomic evaluations in females is to improve the reliability of selection decisions. This will prevent heifers of low genetic value from entering the herd and so increasing genetic gain. Genomic evaluations are useful for the marketing of pedigree heifers, parentage verification which leads to an increased accuracy of mating plans and accurately avoiding breeding carriers of deleterious recessive alleles together.
Given the low cost of genomic evaluations, it is likely that many herds would economically benefit from genotyping some, or all of the herd. The economic gain of using genomic evaluations in a dairy herd increases with the number of decisions the information is used for, the fewer the generations of pedigree information, whether the herd is aiming to breed elite animals and if the herd has seen the widespread use of bulls which are carriers of deleterious recessive alleles.
Herds should only genotype if they expect to recover the cost of testing. The major economic benefit is through selecting a proportion of heifers to enter the herd. If the farm has poor reproductive performance and all heifers will enter the herd regardless of genetic value, it is not worthwhile to conduct genomic evaluations on the herd.

