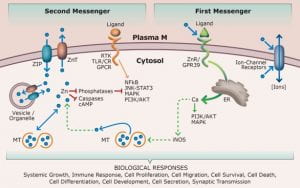
Zinc
Zinc is an essential micronutrient which is now recognized as a signaling molecule in addition to its well-characterized regulatory, catalytic, and structural roles. The maintenance of zinc homeostasis and the targeted cellular function of zinc is facilitated by two families of metal transporters, ZIP and ZnT. ZIP14/SLC39A14 is a ZIP family member, which traffics metals into the cytoplasm. Whole-body knockout (KO) of the Zip14 in mice alters zinc homeostasis and creates a phenotype with key features of metabolic endotoxemia (low-grade chronic inflammation). This signature includes increased intestinal permeability, elevated serum endotoxin levels, increased adiposity coupled with up-regulated cytokine expression and insulin insensitivity in adipose tissue, and enlarged pancreatic islets with greater serum insulin.
(Ryu MS, Aydemir TB. 2019. Chapter 23. Zinc. Present Knowledge in Nutrition: Basic Nutrition and Metabolism, Eleventh Edition. Elsevier)
Using both in vitro cell culture systems and in vivo whole-body and tissue-specific conditional (intestine, pancreatic beta-cells, and liver) Zip14 KO mouse models we aim to answer three main questions:
1- What is the role of ZIP14-mediated zinc transport in intestinal barrier function, specifically in the gut microbiota profile and corresponding host response?
2- What is the role of ZIP14-mediated zinc transport in pancreatic insulin biosynthesis and secretion?
3- What is the role of ZIP14-mediated zinc transport in hepatic glucose metabolism?
Manganese
Manganese (Mn) is an essential micronutrient. Impaired Mn homeostasis results in excess Mn and neurotoxicity. ZIP14/SLC39A14 is a ZIP family transmembrane protein that regulates intracellular manganese and iron in addition to Zn. Recently, it has been shown that humans carrying ZIP14 mutations develop childhood-onset parkinsonism and dystonia. This phenotype was recapitulated in Zip14 knockout (KO) mice. However, the mechanism of ZIP14-mediated Mn detoxification and how defective ZIP14 function leads to Mn overload, systemic and neuroinflammation have not been well-characterized. Using whole-body and intestine-specific Zip14 KO mouse models, we recently identify basolaterally localized ZIP14 as a route of systemic Mn elimination (below figure).
We will further investigate the organ/tissue-specific role of ZIP14-mediated Mn transport in Mn homeostasis. The two aims are:
1- Determine if ZIP14 at the BL membrane of brain endothelial cells regulates Mn detoxification and if defective ZIP14 function leads to neuroinflammation and neurodegeneration.
2- Determine if impaired intestinal barrier function and low-grade chronic inflammation in Zip14 KO mice contribute to ZIP14-associated parkinsonism.