Time-resolved dimerization of a PAS-LOV protein measured with photocoupled small angle x-ray scattering
J. S. Lamb, B. Zoltowski, S. A. Pabit, B. R. Crane, and L. Pollack
J. Amer. Chem. Soc. 130, 12226 (2008).
ABSTRACT
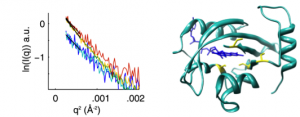
Time-resolved small angle x-ray scattering (SAXS) has been used to probe photoexcitation of the blue-light signal transduction protein Vivid (VVD). Laser excitation of sample in a continuous flow cell reveals initial global dynamics of the long-lived VVD light-state. Good signal-to-noise is achieved without relying on multiple exposures of the same sample or limiting exposure times to prevent radiation damage. The SAXS data demonstrate that VVD oligomerizes within hundreds of milliseconds of light-state activation. Time-resolved SAXS in a flow cell format is a general method for connecting chemical changes in photoreceptors to conformationally driven output signals.
Illuminating solution responses of a LOV-domain protein with photocoupled small angle x-ray scattering
J. S. Lamb, B. Zoltowski, S. Pabit, L. Li, B. Crane, and L. Pollack
Journal of Molecular Biology 393, 909-919 (2009).
ABSTRACT
The PAS-LOV domain is a signal transducing component found in a large variety of proteins that is responsible for sensing different stimuli such as light, oxygen and voltage. The LOV protein VVD regulates blue-light responses in the filamentous fungi Neurospora crassa. Using photocoupled, time-resolved small angle x-ray scattering, we extract the solution protein structure in the wild type protein: a compact structure that corresponds to the crystal structure of the dark-state monomer as well as an extended structure that is well modeled by introducing conformational disorder at the N-terminus of the protein. These conformations are accentuated in carefully selected variants, in which a key residue for propagating structural transitions, Cys71, has been mutated or oxidized. Despite different dark state conformations, all proteins form a common dimer in response to illumination. Taken together, these data support a reaction scheme that describes the mechanism for light-induced dimerization of VVD. Envelope reconstructions of the transient light-state dimer reveal structures that are best described by a parallel arrangement of subunits that have significantly changed conformation compared to the crystal structure.