- Recent research guides management recommendations for 2022
- Recent research guides management recommendations for 2021
This foliar, fungal disease is common wherever cucurbits are grown, including in the northeastern U.S. This is because the pathogen produces an abundance of asexual spores (the powdery growth) easily dispersed by wind, thus it can spread widely, and the pathogen can produce a sexual spore in fall that enables it to survive over winter. It is arguably the most important disease of cucurbits.
Effectively managing powdery mildew is essential for producing a high-quality cucurbit crop. Leaves affected by powdery mildew die prematurely which results in fewer fruit and/or fruit of low quality (poor flavor, sunscald, poor storability). Powdery mildew is managed with resistant varieties and fungicides. Avoiding the pathogen or creating less favorable environment are not viable management practices. An integrated program with both management tools is the best approach for achieving effective control because the pathogen is adept at evolving new strains resistant to individual tools: resistant varieties and specific fungicides. It is more difficult for new pathogen strains to develop when an integrated program is used, and effective control is more likely. Powdery mildew management program often needs adjustments as the pathogen and management tools frequently change.
The Cucurbit Powdery Mildew photo gallery page has additional information as well as photographs.
Research Topics, Results and Reports
Fungicides and resistant varieties have been evaluated through replicated experiments conducted since 1989 under field conditions at the Cornell University research facility on Long Island. These results guide fungicide recommendations for growers.
Topics on this page:
- Fungicide Evaluations – Conventional Products
- Fungicide Resistance – Summary Results
- Fungicide Resistance – In-Field Seedling Bioassays
- Fungicide Resistance – Laboratory Leaf Disk Bioassays
Yearly Results from Leaf Disk Bioassays - Fungicide Evaluations – Biopesticides and other Organic Products
- Fungicide Evaluations – Biopesticides in Programs with Conventional Fungicides
- Nozzle and Air Assist Sprayer Evaluation for Improving Spray Coverage
- Plant Resistance Evaluations – New and Experimental Varieties
- Pathogen Biology
Plant Factors Determining Disease Onset
Sexual Reproduction and Chasmothecial Formation



Fungicide Evaluations – Conventional Products
Fungicide evaluations conducted each year on Halloween-type pumpkin at LIHREC include fungicides at risk for resistance tested alone. This is neither a recommended nor labeled commercial use pattern for these fungicides; it is done in efficacy evaluations to determine inherent activity of new products and if resistance has affected control of products in commercial use. Fungicides are applied weekly using a tractor-sprayer to field-grown pumpkin typically starting after reaching the threshold of 1 of 50 older leaves with symptoms in most plots. Efficacy is assessed by comparing to non-treated plants (control plots) and usually also plants treated with a recommended alternation among labeled fungicides (grower standard without including the recommended protectant fungicide in each application). A focus has been fungicides at risk for resistance because their targeted activity on fungi, the result of having single site mode of action, means they can move through leaves without causing phytotoxicity and provide control on the underside of leaves where the powdery mildew pathogen develops best. This research has documented year-to-year variation in efficacy of some fungicides reflecting occurrence of resistance in the pathogen population. Differences in inherent efficacy among fungicides has also been documented. Fungicides and resistant varieties have been evaluated through replicated experiments conducted since 1989 under field conditions at the Cornell University research facility on Long Island. These results combined with results from research on fungicide resistance (see below) guide fungicide recommendations for growers.
Fungicide evaluations conducted each year on pumpkin at LIHREC on Long Island include fungicides at risk for resistance tested alone (this is neither a labeled nor recommended commercial use pattern for these fungicides; it is done in efficacy evaluations to determine if resistance affects control). Not every fungicide chemistry prone to resistance is tested every year. Efficacy is based on control achieved on lower (under) surface of leaves because this is where the disease develops best. In 2023, Vivando was significantly more effective than Prolivo (both FRAC 50 fungicides): 86% vs 59% control of powdery mildew on lower leaf surfaces. Regev was ineffective. Most effective control (96%) was obtained with Vivando applied in alternation with a DMI fungicide (Proline and Procure) starting after the IPM threshold. In 2022 Cevya was again only moderately effective (54% control on lower leaf surfaces), confirming 2020 and 2021 results, although this time it was tested on an intermediate resistant variety and preventive application schedule, while Prolivo provided 87% control, which was better than in the 2016 experiment perhaps due to these test parameters and using an adjuvant as recommended by the manufacturer. In another experiment with the usual susceptible pumpkin variety, the recommended program of Vivando applied in alternation with Proline and Procure starting after the IPM threshold was reached provided 90% control. In 2021 Cevya was only moderately effective (56% control on lower leaf surfaces), confirming 2020 results, while excellent control (96%) was obtained with Proline alternated with Vivando, a program recommended for Long Island. In 2020 Proline provided exceptional control (99.5% control on lower leaf surfaces); Luna Experience, Quintec, Vivando, and Procure also performed well (89-95%) while Cevya, a new FRAC 3 fungicide, did not (44% at highest rate tested). The FRAC 7 active ingredient in Luna pre-mix fungicides (fluopyram) was shown to be highly effective through testing Luna Privilege, which has only fluopyram; it is not labeled for powdery mildew. In 2019 Vivando was the most effective single product tested providing control statistically similar to the alternation program (Vivando, Quintec, Proline), albeit numerically lower control (75 and 92% control on lower leaf surfaces). Gatten was as effective as Vivando (65%); Luna Sensation and Quintec were less effective (47 and 40%). In 2018 Vivando was most effective albeit not significantly better than Quintec, which was not significantly better than Luna Sensation (54, 42, and 28%). In 2017 Torino, Pristine, and Luna Sensation were ineffective, while Vivando was most effective (80%) albeit not significantly better than Quintec (72%) or Procure (54%). In 2016 Quintec and Procure were as effective as an alternation program (98, 91, and 97%) while Pristine and Prolivo were significantly less effective (43 and 66%). In 2015 Quintec, Pristine, and Vivando were as effective as an alternation program (69-78%). Quintec and Vivando were the most effective of the targeted fungicides evaluated in 2014 (96 and 98%); Pristine was moderately effective (54%); Procure was slightly but not significantly better (70%). In 2013 Quintec, Pristine, and Procure provided excellent control (93-99%). In 2012 Pristine and Fontelis were ineffective (albeit treated pumpkins were numerically less severely affected by powdery mildew than the non-treated plots) while Quintec was very effective (96%) and Procure was moderately effective (57%). These experiments have documented year-to-year variation in the pathogen population. See also table of results from these and previous experiments.
Photographs from experiments: 2023 | 2022 | 2021
Publications (most reports linked here are also available at Plant Disease Management Report website):
- McGrath, M. T. 1990. Effect of timing of Bayleton application(s) on control of pumpkin powdery mildew, 1989. Fungicide and Nematicide Tests 45:130.
- McGrath, M. T. 1991. Evaluation of fungicides and effect of timing of Bayleton applications on control of pumpkin powdery mildew, 1990. Fungicide and Nematicide Tests 46:164.
- McGrath, M. T. 1992. Efficacy of fungicides applied preventatively or based on disease occurrence for managing powdery mildew of pumpkins, 1991. Fungicide and Nematicide Tests 47:124.
- McGrath, M. T. 1992. Fungicides and integrated use of genetic and chemical control for managing powdery mildew of summer squash, 1991. Fungicide and Nematicide Tests 47:133.
- McGrath, M. T., Ghemawat, M. S., and Staniszewska, H. 1993. Efficacy of fungicides applied preventively or following disease detection for managing powdery mildew of pumpkin, 1992. Fungicide and Nematicide Tests 48:173.
- McGrath, M. T., Ghemawat, M. S., and Staniszewska, H. 1993. Fungicides and integrated use of genetic and chemical control for managing powdery mildew of summer squash, 1992. Fungicide and Nematicide Tests 48:183.
- McGrath, M. T., and Staniszewska, H. 1994. Efficacy of fungicides applied preventively or following disease detection for managing powdery mildew of pumpkin, 1993. Fungicide and Nematicide Tests 49:142.
- McGrath, M. T., and Staniszewska, H. 1994. Fungicides and integrated use of genetic and chemical control for managing powdery mildew of crookneck summer squash, 1993. Fungicide and Nematicide Tests 49:149.
- McGrath, M. T., and Staniszewska, H. 1994. Fungicides and integrated use of genetic and chemical control for managing powdery mildew of straightneck summer squash, 1993. Fungicide and Nematicide Tests 49:148.
- McGrath, M. T. 1995. Evaluation of a biocontrol agent and biocompatible fungicides for managing powdery mildew of winter squash, 1994. Biological and Cultural Tests 10:149.
- McGrath, M. T. 1995. Evaluation of full-season and reduced-sprays fungicide programs initiated after disease detection for managing powdery mildew of pumpkin, 1994. Fungicide and Nematicide Tests 50:146-147.
- McGrath, M. T. 1995. Fungicides and integrated use of genetic and chemical control for managing powdery mildew of summer squash, 1994. Fungicide and Nematicide Tests 50:157-158.
- McGrath, M. T. 1996. Evaluation of fungicide programs initiated after disease detection for managing powdery mildew of pumpkin, 1995. Fungicide and Nematicide Tests 51:143-144.
- McGrath, M. T. 1996. Fungicides and integrated use of genetic and chemical control for managing powdery mildew of squash, 1995. Fungicide and Nematicide Tests 51:149-150.
- McGrath, M. T., and Shishkoff, N. 1996. Evaluation of a biocontrol agent and biocompatible fungicides for managing powdery mildew of melon, 1995. Biological and Cultural Tests 11:106.
- McGrath, M. T. 1997. Evaluation of a biocontrol agent and biocompatible fungicides for managing powdery mildew of pumpkin, 1996. Biological and Cultural Tests 12:160.
- McGrath, M. T. 1997. Evaluation of fungicide programs initiated after disease detection for managing powdery mildew of pumpkin, 1996. Fungicide and Nematicide Tests 52:167-168.
- McGrath, M. T., and Shishkoff, N. 1997. Evaluation of a biocontrol agent and biocompatible fungicides for managing powdery mildew of melon, 1996. Biological and Cultural Tests 12:144.
- McGrath, M. T., and Shishkoff, N. 1998. Evaluation of fungicide programs initiated after disease detection for managing powdery mildew of pumpkin, 1997. Fungicide and Nematicide Tests 53:227-228.
- McGrath, M. T., and Shishkoff, N. 1999. Evaluation of fungicide programs for managing powdery mildew of pumpkin, 1998. Fungicide and Nematicide Tests 54:230-231.
- McGrath, M. T. 2000. Evaluation of fungicide programs for managing powdery mildew of pumpkin, 1999. Fungicide and Nematicide Tests 55:253-254.
- McGrath, M. T., and Shishkoff, N. 2000. Evaluation of fungicide programs for managing powdery mildew of muskmelon, 1999. Fungicide and Nematicide Tests 55:176-177.
- McGrath, M. T. 2001. Alternative fungicides to Bravo evaluated on muskmelon cultivars differing in susceptibility to powdery mildew, 2000. Fungicide and Nematicide Tests 56:V75.
- McGrath, M. T. 2001. Alternative fungicides to Bravo evaluated on pumpkin cultivars differing in susceptibility to powdery mildew, 2000. Fungicide and Nematicide Tests 56:V77.
- McGrath, M. T., and Shishkoff, N. 2001. Evaluation of fungicide programs for managing powdery mildew of pumpkin, 2000. Fungicide and Nematicide Tests 56:V76.
- McGrath, M. T. 2002. Evaluation of fungicide programs for managing powdery mildew of pumpkin, 2001. Fungicide and Nematicide Tests 57:V086.
- McGrath, M. T., and Landers, A. 2002. Efficacy of Bravo for powdery mildew control in muskmelon as affected by nozzle type and sprayer, 2001. Fungicide and Nematicide Tests 57:V049.
- McGrath, M. T., and Landers, A. 2002. Efficacy of Bravo for powdery mildew control in pumpkin as affected by nozzle type and sprayer, 2001. Fungicide and Nematicide Tests 57:V085.
- McGrath, M. T. 2002. Alternative fungicides to Bravo evaluated on muskmelon cultivars differing in susceptibility to powdery mildew, 2001. Fungicide and Nematicide Tests 57:V048.
- McGrath, M. T. 2002. Alternative fungicides to Bravo evaluated on pumpkin cultivars differing in susceptibility to powdery mildew, 2001. Fungicide and Nematicide Tests 57:V084.
- McGrath, M. T. 2002. Efficacy of genetic control and chemical control for managing powdery mildew in winter squash, 2001. Biological and Cultural Tests 17:V022.
- McGrath, M. T., and Shishkoff, N. 2003. Evaluation of fungicide programs for managing powdery mildew of pumpkin, 2002. Fungicide and Nematicide Tests 58:V023.
- McGrath, M. T. 2003. Protectant fungicides for managing powdery mildew of pumpkin, 2002. Fungicide and Nematicide Tests 58:V022.
- McGrath, M. T. 2003. Efficacy of genetic control and chemical control for managing powdery mildew in butternut-type winter squash, 2002. Biological and Cultural Tests 18:V016.
- McGrath, M. T. 2003. Efficacy of genetic control, used alone and combined with fungicides, for managing powdery mildew in acorn-type winter squash, 2002. Biological and Cultural Tests 18:V015.
- McGrath, M. T. 2004. Efficacy of Bravo for powdery mildew control in muskmelon and pumpkin as affected by nozzle type and sprayer, 2003. Fungicide and Nematicide Tests 59:V057.
- McGrath, M. T. 2004. Efficacy of genetic control, used alone and combined with fungicides, for managing powdery mildew in acorn-type winter squash, 2003 Biological and Cultural Tests 19:V018.
- McGrath, M. T. 2004. Efficacy of genetic control and chemical control for managing powdery mildew in butternut-type winter squash, 2003. Biological and Cultural Tests 19:V019.
- McGrath, M. T. 2004. Evaluation of biofungicides and compost tea for managing powdery mildew of pumpkin, 2003. Fungicide and Nematicide Tests 59:V055.
- McGrath, M. T. 2004. Evaluation of fungicide programs for managing powdery mildew of pumpkin, 2003. Fungicide and Nematicide Tests 59:V056.
- McGrath, M. T. 2005. Comparison of powdery mildew resistant pumpkin and winter squash under reduced-fungicide program, 2004. Biological and Cultural Tests 20:V011.
- McGrath, M. T. 2005. Evaluation of biofungicides and compost tea for managing powdery mildew of pumpkin, 2004, Fungicide and Nematicide Tests 60:V050.
- McGrath, M. T. 2005. Evaluation of biofungicides for managing powdery mildew of pumpkin, 2004. Fungicide and Nematicide Tests 60:V051.
- McGrath, M. T. 2005. Evaluation of fungicide programs for managing pathogen resistance and powdery mildew of pumpkin, 2004. Fungicide and Nematicide Tests 60:V049.
- McGrath, M. T. 2005. Experimental fungicides compared to fungicides registered for managing powdery mildew of winter squash, 2004. Fungicide and Nematicide Tests 60:V056.
- McGrath, M. T., and Davey, J. F. 2006. Evaluation of biofungicides for managing powdery mildew of pumpkin, 2005. Fungicide and Nematicide Tests 61:V036.
- McGrath, M. T., and Davey, J. F. 2006. Evaluation of copper fungicides for managing powdery mildew of pumpkin, 2005. Fungicide and Nematicide Tests 61:V037.
- McGrath, M. T., and Davey, J. F. 2006. Evaluation of fungicide programs for managing powdery mildew of pumpkin, 2005. Fungicide and Nematicide Tests 61:V038.
- McGrath, M. T., and Davey, J. F. 2006. Experimental fungicide compared to fungicide registered for managing powdery mildew of winter squash, 2005. Fungicide and Nematicide Tests 61:V039.
- McGrath, M. T., and Davey, J. F. 2007. Evaluation of biofungicides for managing powdery mildew of pumpkin, 2006. Plant Disease Management Reports 1:V145.
- McGrath, M. T., and Davey, J. F. 2007. Evaluation of fungicides for management of powdery mildew on pumpkin, 2006. Plant Disease Management Reports 1:V144.
- McGrath, M. T., and Davey, J. F. 2007. Evaluation of fungicides for the management of powdery and downy mildews of winter squash, 2006. Plant Disease Management Reports 1:V148.
- McGrath, M. T., and Fox, G. M. 2008. Evaluation of fungicides for managing powdery mildew in pumpkin, 2007. Plant Disease Management Reports 2:V142.
- McGrath, M. T., and Fox, G. M. 2008. Evaluation of an integrated management program with fungicides and a resistant cultivar for managing powdery mildew in zucchini squash, 2007. Plant Disease Management Reports 2:V148.
- McGrath, M. T., and Fox, G. M. 2008. Evaluation of integrated management programs with biofungicides and a resistant cultivar for managing powdery mildew in butternut squash, 2007. Plant Disease Management Reports 2:V079.
- McGrath, M. T., and Fox, G. M. 2008. Evaluation of integrated management programs with biofungicides and a resistant cultivar for managing powdery mildew in muskmelon, 2007. Plant Disease Management Reports 2:V078.
- McGrath, M. T., and Fox, G. M. 2008. Evaluation of integrated management programs with biofungicides and a resistant cultivar for managing powdery mildew in pumpkin, 2007. Plant Disease Management Reports 2:V141.
- McGrath, M. T., and Fox, G. M. 2009. Efficacy of fungicides for managing cucurbit powdery mildew and treatment impact on pathogen sensitivity to fungicides, 2008. Plant Disease Management Reports 3:V125.
- McGrath, M. T., and Fox, G. M. 2009. Evaluation of a biopesticide (MOI-106) and Rally for managing powdery mildew on pumpkin, 2008. Plant Disease Management Reports 3:V124.
- McGrath, M. T., and Fox, G. M. 2010. Efficacy of fungicides for managing cucurbit powdery mildew and treatment impact on pathogen sensitivity to fungicides, 2009. Plant Disease Management Reports 4:V147.
- McGrath, M. T., and Fox, G. M. 2010. Integrated programs with biopesticides and a resistant cultivar evaluated for powdery mildew in butternut squash, 2009. Plant Disease Management Reports 4:V024.
- McGrath, M. T., and Fox, G. M. 2010. Integrated programs with biopesticides and a resistant cultivar evaluated for powdery mildew in muskmelon, 2009. Plant Disease Management Reports 4:V025.
- McGrath, M. T., and Fox, G. M. 2010. Integrated programs with biopesticides and a resistant cultivar evaluated for powdery mildew in pumpkin, 2009. Plant Disease Management Reports 4:V114.
- McGrath, M. T., and Hunsberger, L. K. 2011. Efficacy of fungicides for managing cucurbit powdery mildew and pathogen sensitivity to fungicides, 2010. Plant Disease Management Reports 5:V104.
- McGrath, M. T., and Hunsberger, L. K. 2011. Efficacy of fungicides for managing powdery mildew in muskmelon, 2010. Plant Disease Management Reports 5:V103.
- McGrath, M. T., and Hunsberger, L. K. 2012. Efficacy of fungicides for managing cucurbit powdery mildew and pathogen sensitivity to fungicides, 2011. Plant Disease Management Reports 6:V080.
- McGrath, M. T., and Hunsberger, L. K. 2012. Efficacy of Vivando for managing powdery mildew in cucurbit crops, 2011. Plant Disease Management Reports 6:V007.
- McGrath, M. T. and LaMarsh, K. A. 2013. Efficacy of fungicides for managing powdery mildew in pumpkin, 2012. Plant Disease Management Reports 7:V013.
- McGrath, M. T. and LaMarsh, K. A. 2014. Comparison of organic and conventional copper fungicides for powdery mildew in zucchini, 2013. Plant Disease Management Reports 8:V193.
- McGrath, M. T. and LaMarsh, K. A. 2014. Efficacy of fungicides for managing powdery mildew in pumpkin, 2013. Plant Disease Management Reports 8:V204.
- McGrath, M. T. and LaMarsh, K. A. 2015. Efficacy of fungicides for managing powdery mildew in pumpkin, 2014. Plant Disease Management Reports 9:V030.
- McGrath, M. T. 2016. Efficacy of fungicides for managing powdery mildew in pumpkin, 2015. Plant Disease Management Reports 10:V040.
- McGrath, M. T. and Sexton, Z. F. 2017. Efficacy of biopesticides for managing powdery mildew in pumpkin, 2016. Plant Disease Management Reports 11:V025.
- McGrath, M. T. and Sexton, Z. F. 2017. Efficacy of fungicides for managing powdery mildew in pumpkin, 2016. Plant Disease Management Reports 11:V024.
- McGrath, M. T. and Sexton, Z. F. 2017. Evaluation of management programs without chlorothalonil for powdery mildew in pumpkin, 2016. Plant Disease Management Reports 11:V026.
- McGrath, M. T. and Sexton, Z. F. 2018. Efficacy of fungicides for managing powdery mildew in pumpkin, 2017. Plant Disease Management Reports 12:V067.
- McGrath, M. T. and Sexton, Z. F. 2018. Evaluation of fungicides to reduce chlorothalonil use for powdery mildew on pumpkins, 2017. Plant Disease Management Reports 12:V073.
- McGrath, M. T. and Sexton, Z. F. 2019. Efficacy of fungicides for managing powdery mildew on pumpkin, 2018. Plant Disease Management Reports 13:V119.
- McGrath, M. T. and Sexton, Z. F. 2019. Evaluation of fungicides to reduce chlorothalonil use for powdery mildew on squash, 2018. Plant Disease Management Reports 13:V122.
- McGrath, M. T. and Sexton, Z. F. 2019. Efficacy for powdery mildew on acorn squash of conventional and organic fungicide programs with biopesticides that induce systemic resistance, 2018. Plant Disease Management Reports 13:V120.
- McGrath, M. T. and Sexton, Z. F. 2019. Efficacy of organic fungicides for managing powdery mildew on acorn squash, 2018. Plant Disease Management Reports 13:V121.
- McGrath, M. T. and Sexton, Z. F. 2020. Efficacy of fungicides for managing powdery mildew on pumpkin, 2019. Plant Disease Management Reports 14:V081.
- McGrath, M. T. and Sexton, Z. F. 2020. Effectiveness of conventional and organic fungicide programs with biopesticides that induce systemic resistance for control of powdery mildew in acorn squash, 2019. Plant Disease Management Reports 14:V078.
- McGrath, M. T. and Downing, C. T. 2021. Efficacy of fungicides for managing powdery mildew in pumpkin, 2020. Plant Disease Management Reports 15:V061.
- McGrath, M. T. and Downing, C. T. 2022. Efficacy of biopesticides applied with conventional fungicides for managing powdery mildew in pumpkin, 2021. Plant Disease Management Reports 16:V111.
- McGrath, M. T. and Downing, C. T. 2022. Efficacy of biopesticides for managing powdery mildew in pumpkin, 2021. Plant Disease Management Reports 16:V110.
- McGrath, M. T. and Downing, C. T. 2023. Evaluation of biopesticides and conventional fungicides for managing powdery mildew on a resistant variety of pumpkin, 2022. Plant Disease Management Reports 17:V061.
- McGrath, M. T. and Downing, C. T. 2023. Evaluation of biopesticides and conventional fungicides for managing powdery mildew on pumpkin, 2022. Plant Disease Management Reports 17:V062.
- McGrath, M. T. and Downing, C. T. 2023. Efficacy of a Phytophthora blight fungicide program for powdery mildew and Phytophthora blight, 2022. Plant Disease Management Reports 17:V063.
- Menasha, S. R., McGrath, M. T. and Downing, C. T. 2024. Evaluation of biopesticides and conventional fungicides for managing powdery mildew on pumpkin, 2023. Plant Disease Management Reports 18:V047.
Fungicide Resistance – Summary Results
Procedures and Reports are below
Resistance to FRAC code 1 fungicides (ex. Topsin M) and FRAC code 11 fungicides (ex. Flint, Quadris) has been found at a high frequency for many years. Both are qualitative type of resistance which means pathogen isolates are either sensitive or resistant to all fungicides in these chemistry groups. None are recommended.
Resistance to FRAC code 7 fungicides has also been found to be common. Resistance is partial among fungicides in this chemistry group which means pathogen isolates are fully resistant to some fungicides (ex. Endura, Pristine, Merivon) but sufficiently sensitive to others (ex. Luna fungicides) that they are able to suppress resistant isolates. This reflects differences in how these fungicides bind to the active site in the fungus.
Resistance to FRAC code 3 fungicides was first detected in 1989 and was associated with control failure of Bayleton, the fungicide in use then. This fungicide has been replaced by several fungicides in this chemistry group that vary in inherent activity but are all more effective than Bayleton reflecting the quantitative type of resistance to this chemistry. The pathogen has not exhibited further development of resistance (increased insensitivity) to FRAC code 3 fungicides thus they remain an important component of fungicide program for cucurbit powdery mildew. Recommended fungicides include Proline, Procure, Rhyme, and Luna Experience which also has a FRAC code 7 ingredient.
Resistance to FRAC code 13 fungicide (Quintec) was first detected in 2015. While detected in pathogen isolates collected at the end of powdery mildew development (September), resistance has not been detected early (July) and Quintec has continued to provide good control of powdery mildew until 2019. This fungicide is recommended used sparingly and early in powdery mildew development.
Resistance to FRAC code U6 fungicide (Torino) was detected in 2017. Detection was associated with control failure in the fungicide efficacy trial at LIHREC. Resistance has been detected since, including early in powdery mildew development in 2019 (July). This fungicide is no longer recommended.
Pathogen isolates with resistance to multiple fungicide chemistry groups have been found. Their presence complicates managing resistance and powdery mildew because applying one fungicide can select for isolates that are also resistant to other fungicides. All of the 2020 isolates found to be resistant to Quintec were also resistant to Torino, Endura, and QoI fungicides.
Resistant isolates have been detected in plantings not treated with any targeted fungicides indicating they can compete with sensitive isolates (no fitness cost to resistance).Resistance has not been detected to FRAC code 50 fungicides (testing done with Vivando).
Fungicide Resistance – In-Field Seedling Bioassays
The bioassay entails treating potted seedlings at about the 3-leaf stage with different fungicide doses, putting them in field locations next to plants naturally affected by powdery mildew for a day, then keeping them in a greenhouse until symptoms develop when upper surface of each leaf is assessed for severity based on percent coverage with visible symptoms. Efficacy of each treatment is assessed by comparing severity to that on non-treated control plants.
Detailed description of procedure.




Summary of results. See publications below for additional information and result tables.
2021. Vivando (FRAC 50), Luna Privilege † (7), and Rally (3) were very effective (96-100% control at full and below label doses) in the second bioassay with seedlings put in commercial and research plantings on 14 September. Torino (U6) and Quintec (13) exhibited good efficacy (78-99% control at highest and below label rates) indicating low frequency of resistance present in the pathogen populations. Resistance to these two fungicides have been detected in the past associated with control failure. These fungicides were not applied at any of the locations. Topsin M (1), Flint Extra (11), and Endura (7) were ineffective indicating a high frequency of resistance to these chemistries, which has been detected commonly in the past. These also were not applied. The first bioassay conducted in August was impacted by high temperatures in the greenhouse.
† Luna Privilege (FRAC code 7) was used rather than Luna Experience or Luna Sensation, which are labeled for cucurbit powdery mildew (but not permitted used on Long Island), because they contain a second active ingredient (FRAC code 3 and 11, respectively) which would confound results.
Publications:
- McGrath, M. T. and Sexton, Z. F. 2018. Fungicide sensitivity of cucurbit powdery mildew pathogen population on Long Island, NY, determined with seedling bioassay, 2017. Plant Disease Management Reports 12:V153.
- McGrath, M. T., Sexton, Z. F., and Nadel, S. 2019. Fungicide sensitivity of cucurbit powdery mildew pathogen populations on Long Island, NY, determined using a seedling bioassay, 2018. Plant Disease Management Reports 13:V161.
- McGrath, M. T. and Sexton, Z. F. 2020. Fungicide sensitivity of cucurbit powdery mildew pathogen populations on Long Island, NY, 2019. Plant Disease Management Reports 14:V079.
- McGrath, M. T. and Downing, C. T. 2021. Fungicide sensitivity of cucurbit powdery mildew pathogen populations on Long Island, NY, 2020. Plant Disease Management Reports 15:V062.
- McGrath, M. T. and Downing, C. T. 2022. Fungicide sensitivity of cucurbit powdery mildew pathogen populations on Long Island, NY, determined using a seedling bioassay, 2021. Plant Disease Management Reports 16:V118.
Fungicide Resistance – Laboratory Leaf Disk Bioassays
Pathogen isolates (individuals) are obtained from single powdery mildew colonies (lesions) on field-grown leaves. They are maintained on cotyledons in petri dishes until tested in a bioassay. For the bioassay, seedlings at the cotyledon stage are treated with different fungicide doses, the next day leaf disks are cut from the cotyledons and placed on water agar in a Petri dish, then the disks are inoculated by transferring spores from a culture to the center of each disk. About 10 and 14 days later severity is assessed on each disk. Typically isolates either do not grow or grow well often not showing obvious reduction in growth compared to non-treated disks
Detailed description of procedure.

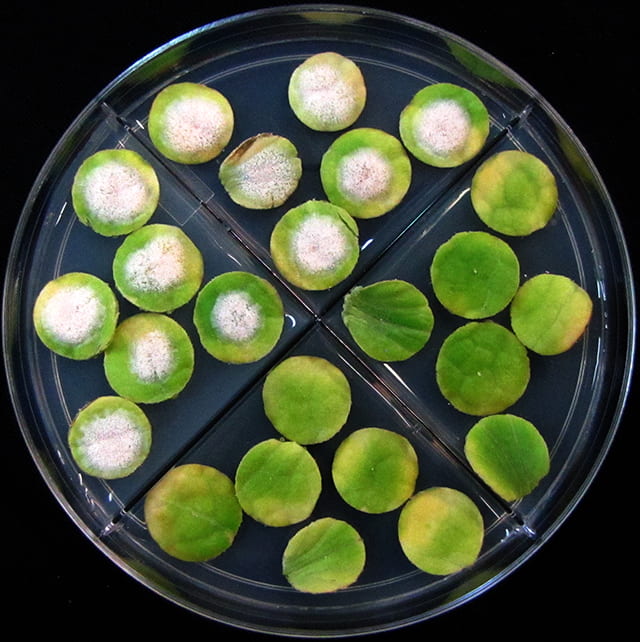
Following are bioassay plates of a sensitive isolate (first) and an isolate with multi-fungicide resistance. They were obtained from pumpkin plants at the end of the 2019 growing season on Long Island.
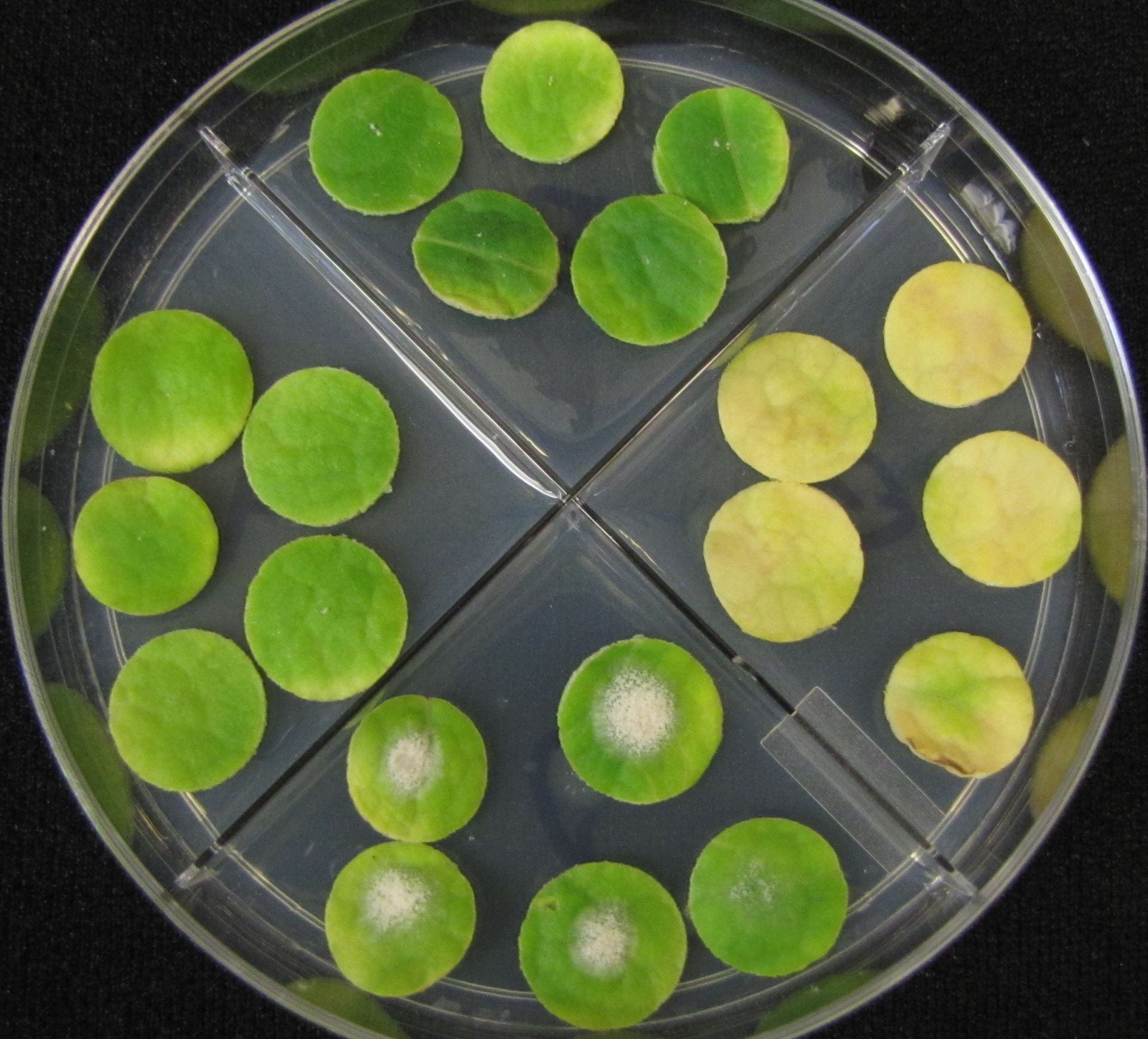

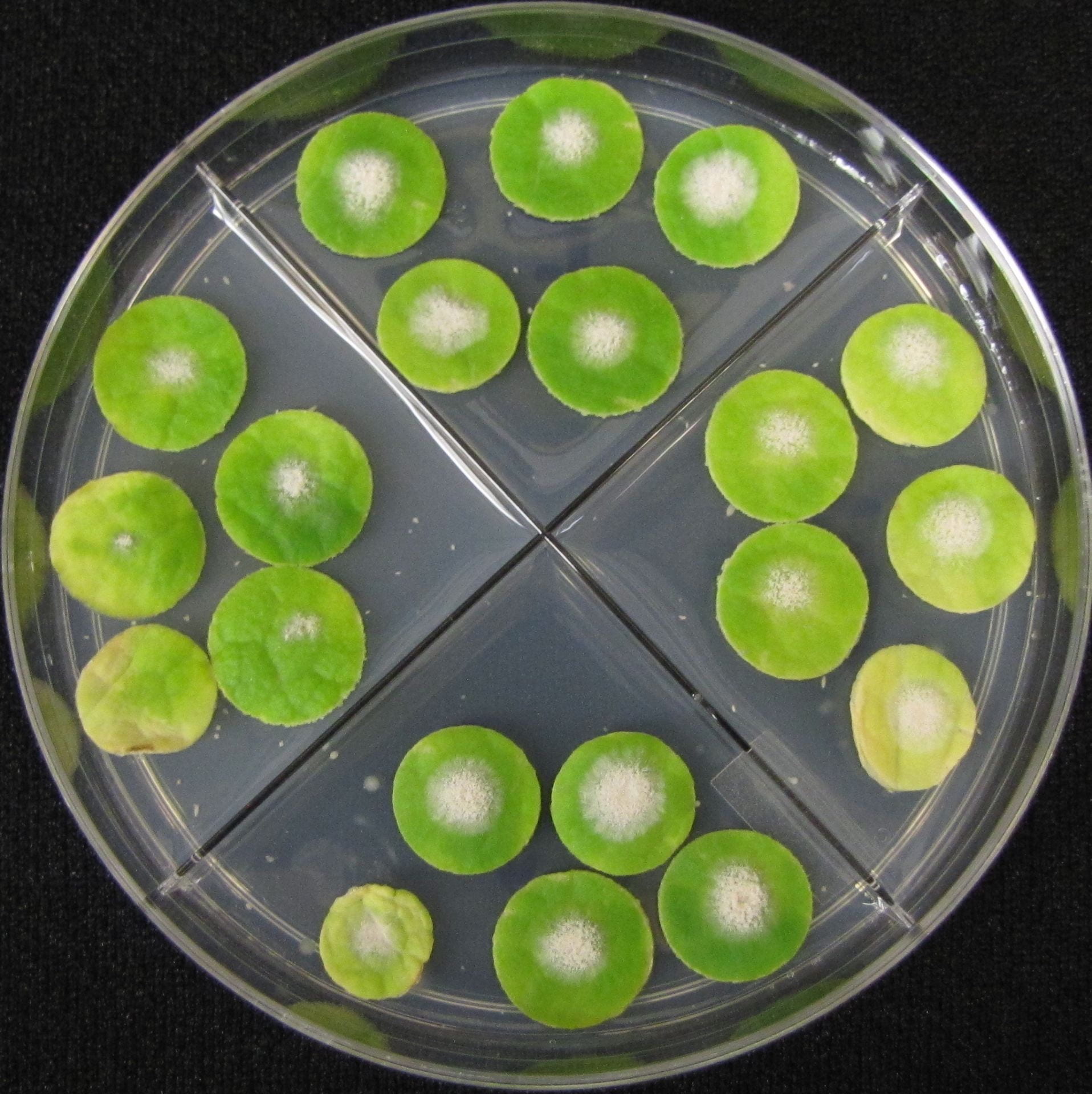
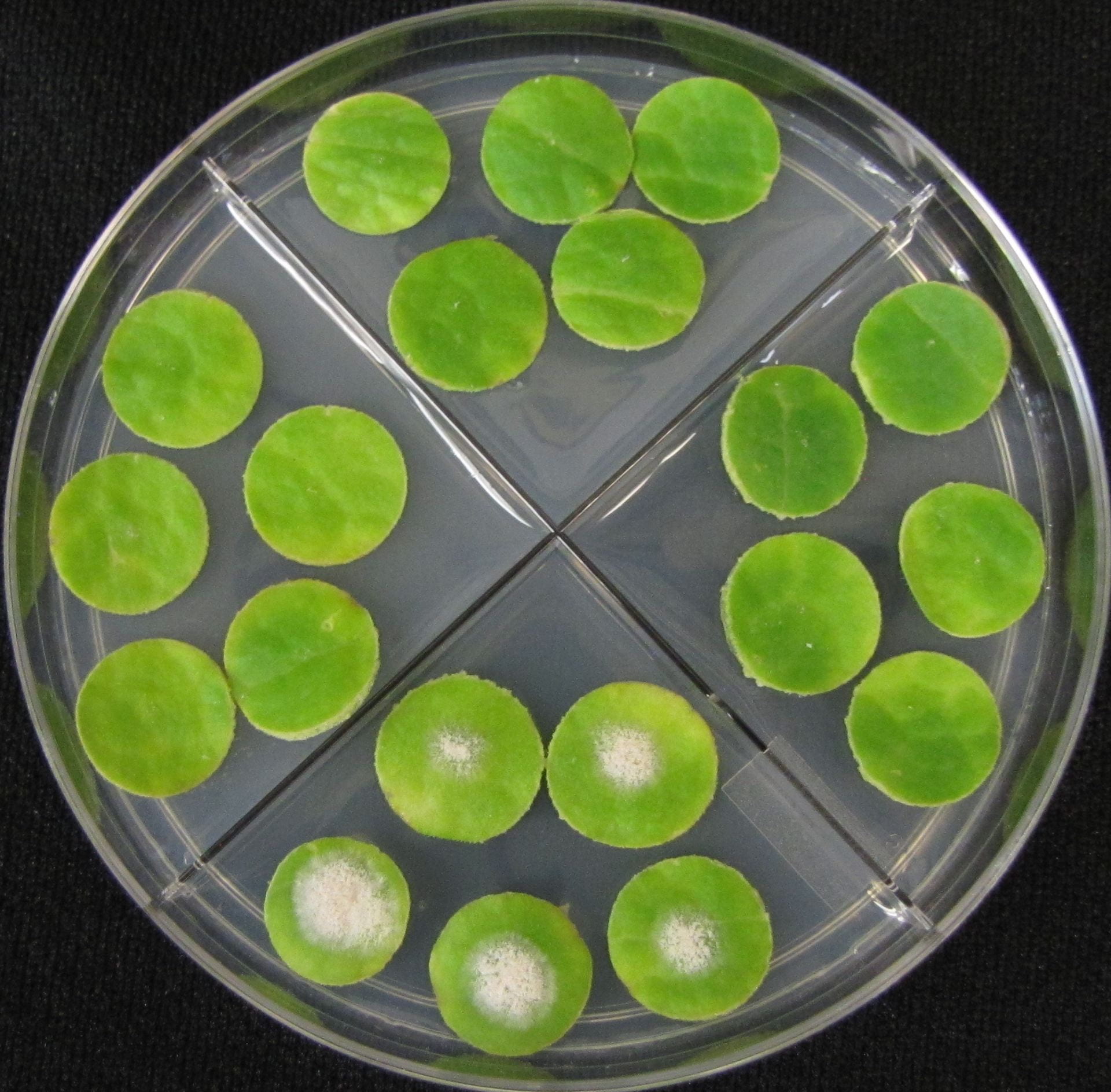
Yearly Results from Testing Isolates for Fungicide Resistance with the Leaf Disk Bioassay
Publications about fungicide resistance:
- McGrath, M. T., and Hausbeck, M. K. 1993. Insensitivity of Sphaerotheca fuliginea to triadimefon in a commercial pumpkin field in Michigan. Plant Disease 77:319.
- McGrath, M. T. 1996. Increased resistance to triadimefon and to benomyl in Sphaerotheca fuliginea populations following fungicide usage over one season. Plant Disease 80:633-639.
- McGrath, M. T, Staniszewska, H., Shishkoff, N., and Casella, G. 1996. Fungicide sensitivity of Sphaerotheca fuliginea populations in the United States. Plant Disease 80:697-703.
- McGrath, M. T., and Shishkoff, N. 2001. Resistance to triadimefon and benomyl: dynamics and impact on control of cucurbit powdery mildew. Plant Disease 85(2):147-154.
- McGrath, M. T. 2001. Fungicide resistance in cucurbit powdery mildew: Experiences and challenges. Plant Disease 85(3):236-245. (Feature article).
- McGrath, M. T., and Shishkoff, N. 2003. First report of the cucurbit powdery mildew fungus (Podosphaera xanthii) resistant to strobilurin fungicides in the United States. Plant Disease 87:1007.
- Lebeda, A., McGrath, M. T., and Sedlákova, B. 2010. Fungicide resistance in cucurbit powdery mildew fungi. Chapter 11. Pages 221-246 In Fungicides. Carisse, O. (Ed.). InTech Publishers, Vienna, Austria. (ISBN 978-953-307-266-1).
- McGrath, M. T. 2011. Challenge of fungicide resistance in managing vegetable diseases in United States and anti-resistance strategies. Chapter 16. Pages 191-207. In Fungicide Resistance in Crop Protection: Threat and Management. Thind, T. S. (Ed.). CABI International, Wallingford, UK.
- McGrath, M. T. 2015. Cucurbit powdery mildew in the USA. Chapter 25. Pages 401-417. In Fungicide Resistance in Plant Pathogens: Principles and a Guide to Practical Management. H. Ishii and D. W. Hollomon (Eds.). Springer Japan KK, Tokyo.
- McGrath, M. T. and Wyenandt, C. A. 2017. First detection of boscalid resistance in Podosphaera xanthii in the United States associated with failure to control cucurbit powdery mildew in New York and New Jersey in 2009. Plant Health Progress 18:93.
- McGrath, M. T. 2017. First report of resistance to quinoxyfen in Podosphaera xanthii, causal agent of cucurbit powdery mildew, in the United States. Plant Health Progress 18:94. [View image]
- Wyenandt, C. A., McGrath, M. T., Everts, K. L., Rideout, S. L., Gugino, B. K., and Kleczewski, N. 2018. Fungicide resistance management guidelines for cucurbit downy and powdery mildew control in the mid-Atlantic and Northeast regions of the United States in 2018. Plant Health Progress 18:1-3.
- McGrath, M. T. and Sexton, Z. F. 2018. Fungicide sensitivity of cucurbit powdery mildew pathogen population on Long Island, NY, determined with seedling bioassay, 2017. Plant Disease Management Reports 12:V153.
- McGrath, M. T. and Sexton, Z. F. 2018. Poor control of cucurbit powdery mildew associated with first detection of resistance to cyflufenamid in the causal agent, Podosphaera xanthii, in the United States. Plant Health Progress 19:222-223. [View image]
- Leadbeater, A., McGrath, M. T., Wyenandt, C.A., and Stevenson, K. L. 2019. An Overview of Fungicide Resistance and Resistance Management – History and Future Trends. Chapter 1. Pages 3-19. In Fungicide Resistance in North America, Second Edition. APS Press, St Paul.
- McGrath, M. T., Sexton, Z. F., and Nadel, S. 2019. Fungicide sensitivity of cucurbit powdery mildew pathogen populations on Long Island, NY, determined using a seedling bioassay, 2018. Plant Disease Management Reports 13:V161.
- McGrath, M. T., Wyenandt, C.A., and Stevenson, K. L. 2019. Occurrence of Fungicide Resistance in Pathogens of Non-Solanaceous Vegetable Crops. Chapter 23. Pages 309-332. In Fungicide Resistance in North America, Second Edition. APS Press, St Paul.
Fungicide Evaluations – Biopesticides and other Organic Products
See: Organic disease management: Powdery mildew in cucurbit crops
Fungicide Evaluations – Biopesticides in Programs with Conventional Fungicides
Below are brief summaries of the main results from each experiment followed by references for reports on each experiment published in Plant Disease Management Reports. Click on the publication year to download the report which has a table of results as well as detailed information about methods and results. Photographs from the experiments and additional information about results are at the linked separate webpages for each experiment.
2023: Based on comparison with the untreated control, all treatments significantly (numerically for 2) improved control of powdery mildew on upper leaf surfaces over that achieved by the general disease maintenance management program applied to the entire experiment (chlorothalonil and/or copper weekly). The three fungicide programs with Theia applied weekly were not significantly better than the parallel program with two applications of a FRAC 50 fungicide (Vivando) and one application of a FRAC 3 fungicide (Proline): 61-72% vs 72% control based on AUDPC values. Regev was ineffective after the 29 Aug assessment. Vivando was significantly more effective than Prolivo based on AUDPC values: 86% vs 59% control; however, there were no significant differences in defoliation or fruit handle quality. The numerically most effective treatment was the grower-recommended program of Vivando applied in alternation with a FRAC 3 fungicide from powdery mildew onset through mid-September (6 applications): 96% control on lower leaf surfaces based on AUDPC values. Effective control on lower leaf surfaces, where powdery mildew develops best, is critical for its successful management. This program also had one of the highest percentages of fruit with good handles.
2022: Powdery mildew was effectively controlled with programs consisting of 2 preventive applications of a biopesticide (Serifel, Theia, or Timorex ACT), 3 applications of targeted fungicides (Proline, Vivando, Proline) started when threshold reached, then 3 applications of the biopesticide. For more information see: Evaluation of Biopesticides in Programs with Conventional Fungicides, 2022
2021: Powdery mildew was effectively controlled with programs consisting of 1 preventive application of a biopesticide (Sil-Matrix, Howler, or Theia), then 3 applications of targeted fungicides (Proline, Vivando, Proline) started when threshold reached and alternated with 3 applications of the biopesticide (two alternation orders tested). The last application was the biopesticide. For more information see: Evaluation of Biopesticides in Programs with Conventional Fungicides, 2021
Publications on evaluations of programs with biopesticides and conventional fungicides:
- McGrath, M. T. and Downing, C. T. 2022. Efficacy of biopesticides applied with conventional fungicides for managing powdery mildew in pumpkin, 2021. Plant Disease Management Reports 16:V111.
- McGrath, M. T. and Downing, C. T. 2023. Evaluation of biopesticides and conventional fungicides for managing powdery mildew on a resistant variety of pumpkin, 2022. Plant Disease Management Reports 17:V061.
- McGrath, M. T. and Downing, C. T. 2023. Evaluation of biopesticides and conventional fungicides for managing powdery mildew on pumpkin, 2022. Plant Disease Management Reports 17:V062.
- Menasha, S. R., McGrath, M. T. and Downing, C. T. 2024. Evaluation of biopesticides and conventional fungicides for managing powdery mildew on pumpkin, 2023. Plant Disease Management Reports 18:V047.
Nozzle and Air Assist Sprayer Evaluation for Improving Spray Coverage
Effectively managing cucurbit powdery mildew necessitates getting fungicide to the lower surface of leaves where the pathogen develops best. One way to accomplish this is by selecting nozzle type and sprayer capable of delivering spray material directly there. Water sensitive paper is a good tool for assessing coverage. Another way is with modern conventional fungicides that have targeted activity and can move into leaf tissue and redistribute including to the lower surface (translaminar movement). However, their single-site mode of action makes them prone to resistance developing in the pathogen, rendering the fungicide ineffective. The cucurbit powdery mildew fungus has a long history of developing resistance. A common recommendation for delaying resistance development is applying fungicides at risk for resistance with contact, protectant fungicides like chlorothalonil and biopesticides because they have low risk due to their multi-site mode of action. To manage resistance most effectively, these fungicides need to be delivered directly to the lower surface of leaves.






Images below: Tractor-drawn sprayer used for research with an air assist boom, a separate hydraulic boom set-up with multiple nozzles on a single nozzle body (visible in fourth image), and five tanks for fungicide treatments plus a water tank. All nozzles tested are in the last image.
The goal of research conducted in 2001 and 2003 was to determine if control of powdery mildew on lower surface of leaves achieved with weekly applications of a contact fungicide (Bravo) could be improved by using an air assist boom or novel nozzles on a standard boom. Adjacent experiments were conducted with cantaloupe and pumpkin. Two novel nozzles (twin jet and air induction) were compared to 3 traditional nozzles (flat fan, hollow cone, and cone jet). All are considered ideal for applying fungicides. Twin-jet nozzles use forward and rearward pointing flat fans, thus providing two chances to hit the plant. They apply the same total amount of liquid as a conventional flat fan. Air induction (air inclusion or venturi) nozzles are flat fan nozzles where an internal venturi creates negative pressure inside the nozzle body. Air is drawn in through two holes in the nozzle side, mixing with the spray liquid. The emitted spray contains large (450 micron) droplets filled with air bubbles and virtually no fine, drift-prone droplets. Normally droplets this large would bounce off their target. However, because of the air bubbles they explode on impact and spread over the leaf as the air absorbs the impact load. Coverage is similar to that of finer sprays produced by traditional nozzles. For comparison, there was a treatment with mobile (translaminar) fungicides applied weekly with a flat fan nozzle.
Results. Neither the air assist boom nor the novel nozzles improved control achieved with Bravo applied with conventional nozzles. Water sensitive paper documented very little spray was deposited on the lower surface of leaves with all nozzles. More effective control of powdery mildew was achieved with the mobile fungicides.
Publications listed below have more information about research results summarized above, including table with results. To download report click on publication year, which is year after study was done; all are available at Plant Disease Management Reports website.
- McGrath, M. T., and Landers, A. 2002. Efficacy of Bravo for powdery mildew control in muskmelon as affected by nozzle type and sprayer, 2001. Fungicide and Nematicide Tests 57:V049.
- McGrath, M. T., and Landers, A. 2002. Efficacy of Bravo for powdery mildew control in pumpkin as affected by nozzle type and sprayer, 2001. Fungicide and Nematicide Tests 57:V085.
- McGrath, M. T. 2004. Efficacy of Bravo for powdery mildew control in muskmelon and pumpkin as affected by nozzle type and sprayer, 2003. Fungicide and Nematicide Tests 59:V057
Plant Resistance Evaluations – New and Experimental Varieties
Evaluations are listed by year conducted followed by the powdery mildew resistant (PMR) varieties tested and the susceptible variety they were compared to. There is more information about the experiments at the linked webpages, which also have photographs, and the reports, which also have data tables.
Cantaloupe
2021: Edisto 47 and Trifecta, which are also resistant to downy mildew, Ambrosia, and Sugar Rush. All PMR varieties exhibited excellent PM suppression and their fruit had substantially higher sugar content than the susceptible variety, Hales Best. Trifecta rated highest for overall fruit quality. For more information see: Evaluation of Cantaloupe Varieties Resistant to Powdery Mildew, 2021 | Cantaloupe Report
2020: Ambrosia, Astound, Halona, Shockwave, Sugar Cube, and Sugar Rush. All PMR varieties exhibited excellent PM suppression. Shockwave produced fruit with highest sugar content; Ambrosia and Astound had significantly lower Brix values. For more information see: Evaluation of New Cantaloupe Varieties Resistant to Powdery Mildew, 2020 | Cantaloupe Report
Pumpkin
2017: Bayhorse Gold, Eagle City Gold, Eureka, Magnum, Millionaire, Progress, and Skidoo Gold compared to Gold Challenger. Progress and Skidoo Gold performed best, suppressing powdery mildew development on upper and lower leaf surfaces. Eureka and Magnum had significantly less severe symptoms on lower leaf surfaces. Bayhorse Gold, Eagle City Gold, and Millionaire were numerically but not significantly less severely affected than Gold Challenger. Fungicides need to be applied to resistant varieties to achieve a commercially-acceptable level of control. For more information see: Evaluation of Halloween Pumpkin Varieties Resistant to Powdery Mildew, 2017 | Pumpkin Report
2016: Ares, Bayhorse Gold, Eagle City Gold, Kratos, Progress, Rhea, and Superior compared to Gold Challenger. Best suppression was obtained with Progress, which has homozygous resistance (resistant gene from both parents). For the others, PM was numerically but not significantly less severe on both leaf surfaces compared to susceptible Gold Challenger. Best control was achieved by applying fungicides to resistant varieties. For more information see: Evaluation of Halloween Pumpkin Varieties Resistant to Powdery Mildew, 2016 | Pumpkin Report
Winter squash – acorn
2020: Autumn Delight, Starry Night, Sugar Bush and Tiptop compared to Table Ace. All PMR varieties exhibited good PM suppression and consequently less defoliation. Fruit of Starry Night had significantly higher sugar content than all other varieties except Sugar Bush, while Autumn Delight had the lowest Brix values. For more information see: Evaluation of New Acorn Squash Varieties Resistant to Powdery Mildew, 2020 | Acorn Squash Report
2019: Sugar Bush and Autumn Delight compared to Table Ace. The 2 PMR varieties exhibited little PM suppression. Sugar Bush had significantly higher sugar content (Brix) than the others. Brix values were generally higher for fruit from plants treated weekly with PM fungicides than untreated suggesting an improvement in fruit quality with excellent PM control achieved with fungicides. For more information see: Evaluation of New Acorn Squash Variety Resistant to Powdery Mildew, 2019 | Acorn Squash Report
Winter squash – butternut
2019: Gabrielle and Quantum compared to Waltham. PM suppression achieved with the 2 PMR varieties alone was good and excellent combined with weekly PM fungicide applications. For more information see: Evaluation of New Butternut Squash Variety Resistant to Powdery Mildew, 2019 | Butternut Squash Report
Publications about resistant variety evaluations (click on publication year to download report; all are available at Plant Disease Management Reports website):
- McGrath, M. T., and Fox, G. M. 2009. Susceptibility to downy mildew of pickling-type cucumber cultivars, 2008. Plant Disease Management Reports 3:V114.
- McGrath, M. T., and Fox, G. M. 2009. Susceptibility to downy mildew of slicer-type cucumber cultivars, 2008. Plant Disease Management Reports 3:V115.
- McGrath, M. T., and Fox, G. M. 2010. Susceptibility to downy mildew of pickling-type cucumber cultivars, 2009. Plant Disease Management Reports 4:V117.
- McGrath, M. T., and Fox, G. M. 2010. Susceptibility to downy mildew of slicer-type cucumber cultivars, 2009. Plant Disease Management Reports 4:V121.
- McGrath, M. T., Menasha, S. R., and LaMarsh, K. A. 2013. Evaluation of downy mildew resistance in experimental hybrids of cucumber, 2012. Plant Disease Management Reports 7:V018.
- McGrath, M. T. and LaMarsh, K. A. 2015. Evaluation of downy mildew resistance in experimental hybrids of cucumber, 2014. Plant Disease Management Reports 9:V024.
- McGrath, M. T., Menasha, S. R., and Sexton, Z. F. 2018. Evaluation of cucumber cultivars resistant to downy mildew, 2017. Plant Disease Management Reports 12:V069.
- McGrath, M. T. and Downing, C. T. 2022. Evaluation of downy mildew resistant cultivars of cantaloupe, 2021. Plant Disease Management Reports 16:V104.
- McGrath, M. T. and Downing, C. T. 2022. Evaluation of downy mildew resistant cultivars of cucumber, 2021. Plant Disease Management Reports 16:V102.
- McGrath, M. T. and Downing, C. T. 2023. Evaluation of a fungicide program applied on an IPM schedule to downy mildew susceptible and resistant cultivars of cantaloupe, 2022. Plant Disease Management Reports 17:V060.