The Asian longhorned beetle (ALB), Anoplophora glabripennis, is a wood-borer in the Cerambycidae, originally native to China and Korea. The species only became widely known as a virulent tree-killer after highly susceptible hybrid poplar trees were extensively planted across China in cities, farms and plantations. The poplars became infested and ALB was subsequently transported to other countries via accidental shipping of infested wood. It was first found in New York State in 1996 and since then has been found in 4 more states in the USA, as well as in Canada and 6 European countries.
The Asian longhorned beetle (ALB), Anoplophora glabripennis, is a wood-borer in the Cerambycidae, originally native to China and Korea. The species only became widely known as a virulent tree-killer after highly susceptible hybrid poplar trees were extensively planted across China in cities, farms and plantations. The poplars became infested and ALB was subsequently transported to other countries via accidental shipping of infested wood. It was first found in New York State in 1996 and since then has been found in 4 more states in the USA, as well as in Canada and 6 European countries.
Our laboratory has been working on methods for biological control of Asian longhorned beetles using entomopathogenic fungi, especially Metarhizium brunneum F52 which is already registered with the EPA for other insects in the U.S. We worked on adapting a product with a novel application strategy (fungal bands; see below) that is used for control of longhorned beetles in Japan. Because populations of ALB are at low densities in the U.S. we gained results demonstrating control through several years of field trials with fungal bands in China. However, this was difficult because reliable beetle populations for field studies were not easy to locate, so we have used quarantine laboratory research at Cornell University to develop effective methods to reduce oviposition and cause mortality of adult beetles. ALB is presently being quarantined and eradicated wherever it is found in the USA. The current USDA program is based on detection, removal of infested trees, and chemical pesticides for prevention. We see the biological controls we are developing as a potential substitute for, or addition to, the chemical insecticides presently in use for eradication.
 Life history & hosts: ALB has the potential for major impacts, due to its wide range of host trees, including species of maple, willow, poplar and aspen, birch, elm, and others. In summer and early fall, the adults feed on twig bark, leaves and petioles, and the females chew oviposition pits in the bark of branches and tree trunks. The primary damage, however, is from the larvae, which feed at first beneath the bark, and then tunnel deeper into the wood, eventually causing branch breakage and even tree death. The larvae take one or more years to reach adulthood. ALB does not kill trees in a single season, but generation after generation of beetles attack the same tree, resulting in progressive death of branches and eventually the tree.
Life history & hosts: ALB has the potential for major impacts, due to its wide range of host trees, including species of maple, willow, poplar and aspen, birch, elm, and others. In summer and early fall, the adults feed on twig bark, leaves and petioles, and the females chew oviposition pits in the bark of branches and tree trunks. The primary damage, however, is from the larvae, which feed at first beneath the bark, and then tunnel deeper into the wood, eventually causing branch breakage and even tree death. The larvae take one or more years to reach adulthood. ALB does not kill trees in a single season, but generation after generation of beetles attack the same tree, resulting in progressive death of branches and eventually the tree.
Infestation history: In China where this species is native, widespread plantings of a susceptible Populus hybrid became infested. This wood-borer was then transported to other countries, often in dunnage within shipping containers. Infestations have been found in North America, Europe (including Germany, Austria, Italy, France, The Netherlands and U.K) and elsewhere. It has been successfully eradicated in several places (The Netherlands, Toronto in Canada, and some sites in the USA), but new populations continue to be discovered. In the USA, it has also been found in warehouses in many locations. Most infested trees have been discovered in urban areas, except in Ohio. The relatively slow spread of this beetle from an infestation has made eradication possible in two U.S. states and hopeful for others. However, it has now been found in forests, and at additional sites in a few regions where it had been thought eradicated. The USDA eradication program for ALB includes inspection of trees (often by climbers), removal and chipping of infested trees, and/or injection of infested trees with the insecticide imidacloprid (the latter case is infrequent); and removal or injection of nearby high-risk host tree species.
Known populations (as of summer 2013) in North America: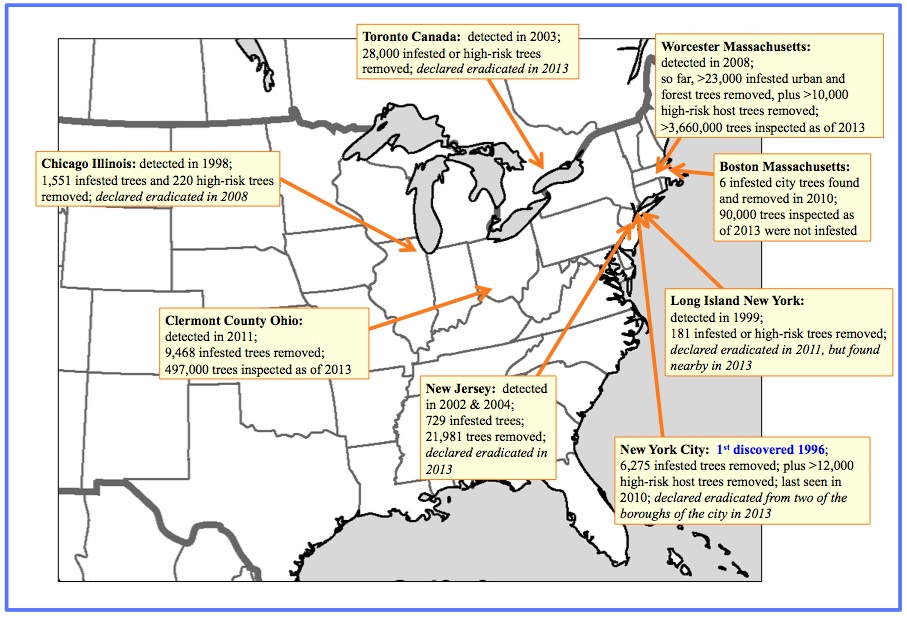
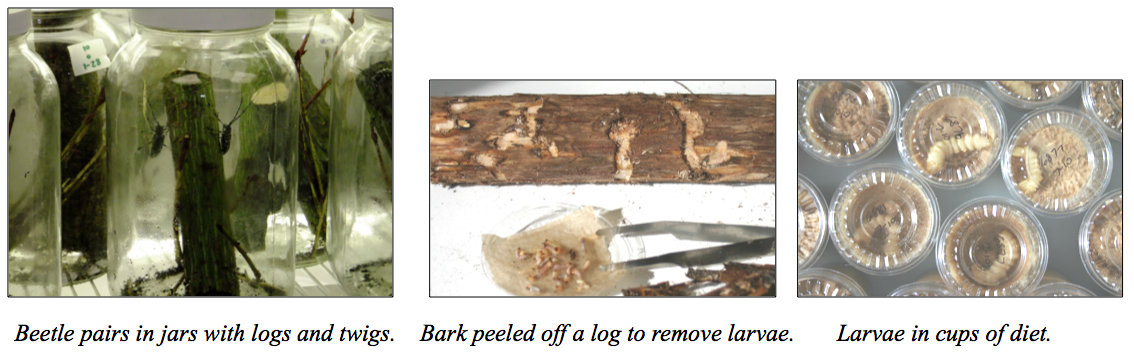
Rearing in quarantine: We have been rearing beetles for use in experiments since 1998, in the laboratory under quarantine protocols. Pairs of beetles are given an oviposition log and twigs to feed on. After a few weeks for the eggs to hatch, the bark is peeled off and young larvae are removed. Larvae are reared in individual containers on artificial diet (primarily composed of cellulose, soy flour, and nutrients), and after several months, are given a 10-12 week chilling period. Nine months are required from oviposition to adulthood in the lab at 23°C. Longevity of adults in the laboratory is generally 3 to 4 months for females, and longer for males. One to three eggs are laid per day, at 23°C.
 Biological control with entomopathogenic fungus: Commercially produced ‘fungal bands’ are used for control of other species of longhorned beetles on orchard trees in Japan. To make fungal bands, material is soaked in agar and fungal culture and incubated; the fungus produces conidia (spores) on the band surface and bands are attached around tree branches or trunks. Beetles that walk over fungal bands made with Metarhizium brunneum become contaminated with a lethal dose. Field studies with fungal bands in China, and laboratory studies in quarantine at Cornell, have demonstrated reduced fecundity before adult death from infection. Conidia can also be spread by contact with mates, to infect beetles that do not encounter fungal bands. This insect-pathogenic fungus does not spread as an endemic or epidemic disease throughout the population, so application of conidia to the beetles is a key step in using this form of biological control. Up to a point, the higher the conidial dose, the faster the beetles can be killed.
Biological control with entomopathogenic fungus: Commercially produced ‘fungal bands’ are used for control of other species of longhorned beetles on orchard trees in Japan. To make fungal bands, material is soaked in agar and fungal culture and incubated; the fungus produces conidia (spores) on the band surface and bands are attached around tree branches or trunks. Beetles that walk over fungal bands made with Metarhizium brunneum become contaminated with a lethal dose. Field studies with fungal bands in China, and laboratory studies in quarantine at Cornell, have demonstrated reduced fecundity before adult death from infection. Conidia can also be spread by contact with mates, to infect beetles that do not encounter fungal bands. This insect-pathogenic fungus does not spread as an endemic or epidemic disease throughout the population, so application of conidia to the beetles is a key step in using this form of biological control. Up to a point, the higher the conidial dose, the faster the beetles can be killed.
Effect of imidacloprid insecticide on ALB adults: In locations in the USA where it is actively being eradicated, the primary alternative to tree removal and means for prevention has been injection of the systemic insecticide imidacloprid. In quarantine experiments, adult ALB were killed when they ingested dissolved imidacloprid at >10 ppm, contacted 50 ppm imidacloprid residue, or ingested twig bark from injected trees with >0 ppm imidacloprid. For beetle pairs fed twigs with >1 ppm, lifetime fecundity was reduced to an average of 2 eggs. Unfortunately the majority of 19 sampled trees that had been injected once in spring 2010 in Worcester, Massachusetts had too little imidacloprid in the twigs or leaves in the summer to have much impact on adults. Quarantine studies have demonstrated a synergistic effect between imidacloprid and Metarhizium brunneum fungal infection, with survival times reduced for beetles exposed to both mortality agents.
Ongoing research: Because greater spore loads result in faster times to death, we have been working in the lab on improving ways to expose beetles to M. brunneum. The goal is to find an application method for trees that would result in large numbers of viable conidia of M. brunneum that would be picked up by the beetles walking on the branches for oviposition and feeding. The focus in 2013-2017 has been using fungal microsclerotia prepared as dry pellets, which upon rewetting are able to grow hyphae and produce infective conidia, over a period of weeks. In a slurry with ingredients to improve moisture-holding and adhesion to the surface, these microsclerotia can be applied to tree bark directly.
Funding for our research has been provided by the Litwin Foundation (New York) and the Alphawood Foundation (Chicago).
All photos © 2013, Hajek Lab.
ALB PUBLICATIONS FROM THE HAJEK LAB
Application of Fungal Microsclerotia Against ALB
Clifton, E.H., Gardescu, S., Behle, R.W., Hajek, A.E. (In press). Optimizing application rates of Metarhizium brunneum microsclerotia for infecting the invasive Asian longhorned beetle (Coleoptera: Cerambycidae). Journal of Economic Entomology.
Clifton, E.H., Gardescu, S., Behle, R.W., Hajek, A.E. May 2019. Asian longhorned beetle bioassays to evaluate formulation and dose-response effects of Metarhizium microsclerotia. Journal of Invertebrate Pathology 163: 64-66. https://doi.org/10.1016/j.jip.2019.03.005
Gardescu, S., Hajek, A.E., Goble T.A., Jackson, M.A. 2017. Metarhizium microsclerotia and hydrogel versus hydromulch: testing fungal formulations against Asian longhorned beetles, Biocontrol Science and Technology 27: 918-930. DOI: 10.1080/09583157.2017.1362546
Goble, T.A., Gardescu, S., Jackson, M.A., Hajek, A.E. 2017. Evaluating Metarhizium brunneum F52 microsclerotia in hydromulch formulations using different tackifiers under forest and orchard conditions. BioControl 62: 769-778. DOI: 10.1007/s10526-017-9835-7
Goble, T.A., Gardescu, S., Fisher, J.J., Jackson, M.A., Hajek, A.E. 2016. Conidial production, persistence and pathogenicity of hydromulch formulations of Metarhizium brunneum F52 microsclerotia under forest conditions. Biological Control 95: 83–93.
Goble, T.A., Gardescu, S., Jackson, M.A., Hajek, A.E. 2016. Evaluating different carriers of Metarhizium brunneum F52 microsclerotia for control of adult Asian longhorned beetles (Coleoptera: Cerambycidae). Biocontrol Science and Technology 26 (9): 1212-1229.
Goble, T.A., Hajek, A.E., Jackson, M.A., Gardescu, S. 2015. Microsclerotia of Metarhizium brunneum F52 applied in hydromulch for control of Asian longhorned beetles (Coleoptera: Cerambycidae). Journal of Economic Entomology 108:433-443.
(Note: includes in Supplementary Data [online], a detailed summary of our current ALB rearing methods)
Developing Fungal Bands
Ugine, T.A., Peters, K.E., Gardescu, S., Hajek, A.E. 2014. The effect of time postexposure and sex on horizontal transmission of Metarhizium brunneum conidia between Asian longhorned beetle (Coleoptera: Cerambycidae) mates. Environmental Entomology 43: 1552-1560.
Ugine, T.A., Jenkins, N., Gardescu, S., Hajek, A.E. 2013. Comparing fungal band formulations for Asian longhorned beetle biological control. J. Invertebr. Pathol. 113: 240-246.
Ugine, T.A., Jenkins, N., Gardescu, S., Hajek, A.E. 2013. Conidial acquisition and survivorship of adult Asian longhorned beetles exposed to flat versus shaggy agar fungal bands. J. Invertebr. Pathol. 113: 247-250.
Shanley, R.P., J. Leland, M. Keena, M.M. Wheeler, A.E. Hajek. 2009. Evaluating the virulence and longevity of non-woven fiber bands impregnated with Metarhizium anisopliae against the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Biol. Control 50: 94-102.
Shanley, R.P., A.E. Hajek. 2008. Environmental contamination with Metarhizium anisopliae from fungal bands for control of the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Biocontr. Sci. Technol. 18: 109-120.
Hajek, A.E., T. Dubois, J. Lund, M. Smith, L. Bauer, and Z. Li. 2007. Developing fungal bands for control of Asian longhorned beetle, Anoplophora glabripennis, in the U.S. J. Anhui Agric. Univ. 34: 149-156. [Invited review].
Hajek, A.E., B. Huang, T. Dubois, M. T. Smith, and Z. Li. 2006. Field studies of control of Anoplophora glabripennis (Coleoptera: Cerambycidae) using fiber bands containing the entomopathogenic fungi Metarhizium anisopliae and Beauveria brongniartii. Biocontr. Sci. Technol. 16: 329-343.
Dubois, T., Z. Li, H. Jiafu, and A.E. Hajek. 2004. Efficacy of fiber bands impregnated with Beauveria brongniartii cultures against the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Biol. Contr. 31: 320-328.
Dubois, T., A.E. Hajek, H. Jiafu, and Z. Li. 2004. Evaluating the efficiency of entomopathogenic fungi against the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae), by using cages in the field. Environ. Entomol. 33: 62-74.
Effects of Fungal Pathogens on ALB
Fisher, J.J., Hajek, A.E. 2015. Maternal exposure of a beetle to pathogens protects offspring against fungal disease. PLOS One. DOI: 10.1371/journal.pone.0125197
Goble, T.A., Rehner, S.A., Long, S.J., Gardescu, S., Hajek, A.E. 2014. Comparing the virulence of North American Beauveria brongniartii and commercial pathogenic fungi against Asian longhorned beetles. Biological Control 72: 91-97.
Fisher, J.J., Hajek, A.E. 2014. Thermoregulatory behavior and fungal infection of the Asian longhorned beetle, Anoplophora glabripennis. Environmental Entomology 43: 384-392.
Peng, F., Gardescu, S., Hajek, A.E. 2011. Transmission of Metarhizium brunneum conidia between male and female Anoplophora glabripennis adults. BioControl 56:771–780.
Hajek, A.E., J. Lund, M.T. Smith. 2008. Reduction in fitness of female Asian longhorned beetle (Anoplophora glabripennis) infected with Metarhizium anisopliae. J. Invertebr. Pathol. 98: 198-205.
Dubois, T., J. Lund, L.S. Bauer, A.E. Hajek. 2008. Virulence of entomopathogenic hypocrealean fungi infecting Anoplophora glabripennis. BioControl 53: 517-528.
Imidacloprid Injected into Trees and Interactions between Imidacloprid and Fungal Entomopathogens
Fisher, J.J., Castrillo, L.A., Donzelli, B.G.G., Hajek, A.E. 2017. Starvation and imidacloprid exposure influence immune response by Anoplophora glabripennis (Coleoptera: Cerambycidae) to a fungal pathogen. Journal of Economic Entomology 110: 1451-1459. DOI: 10.1093/jee/tox124
Ugine, T.A., Gardescu, S.A., Hajek, A.E. 2013. The within-season and between tree distribution of imidacloprid trunk-injected into Acer platanoides. J. Econ. Entomol. 106: 874-882.
Ugine, T.A., Gardescu, S., Lewis, P.A., Hajek, A.E. 2012. Efficacy of imidacloprid, trunk-injected into Acer platanoides, for control of adult Asian longhorned beetles (Coleoptera: Cerambycidae). J. Econ. Entomol. 105: 2015-2028.
Ugine, T., Gardescu, S., Hajek, A.E. 2011. The effect of exposure to imidacloprid on Asian longhorned beetle (Coleoptera: Cerambycidae) survival and reproduction. J. Econ. Entomol. 104: 1942-1949.
Russell, C., Ugine, T.A., Hajek A.E. 2010. Interactions between imidacloprid and Metarhizium brunneum on adult Asian longhorned beetle (Anoplophora glabripennis (Motschulsky)). J. Invertebr. Pathol. 105: 305-311.
General Biology and Reviews
Hu, J., Angeli, S., Schuetz, S., Luo, Y., Hajek, A.E. 2009. Ecology and management of exotic and endemic Anoplophora glabripennis (Coleoptera: Cerambycidae). Agric. For. Entomol. 11: 359-375.
Hajek, A.E., L.S. Bauer. 2009. Use of entomopathogens against invasive wood boring beetles in North America, pp. 159-179. In: A.E. Hajek, T.R. Glare & M. O’Callaghan (eds.) Use of Microbes for Control and Eradication of Invasive Arthropods. Springer, Dordrecht, NL.
Hajek, A.E., D.M. Kalb. 2007. Suitability of Acer saccharum and Acer pensylvanicum for rearing Anoplophora glabripennis (Coleoptera: Cerambycidae). Can. Entomol. 139: 751-755.
Hajek, A.E., and L. S. Bauer. 2007. Microbial control of wood-boring insects attacking forest and shade trees, pp. 505-525. In (L.A. Lacey & H.K. Kaya, Eds.) Field Manual of Techniques in Invertebrate Pathology, 2nd edn. Springer, Dordrecht, Netherlands [Invited review].
Hajek, A.E. 2007. Asian longhorned beetle: Ecology and control, pp. 21-24. In (D. Pimentel, Ed.) Encyclopedia of Pest Management, Vol. II. CRC Press, Taylor & Francis Group, Boca Raton, FL
Hajek, A.E., R.T. Curtiss, J.K. Liebherr. 2004. Characters differentiating male from female Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae). Proc. Entomol. Soc. Wash. 106: 928-931.
Dubois, T., A.E. Hajek, and S. Smith. 2002. Methods for rearing the Asian longhorned beetle (Coleoptera: Cerambycidae) on artificial diet. Ann. Entomol. Soc. Amer. 95: 223-230.